UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
|
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended
or
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number:
(Exact name of registrant as specified in its charter)
|
|
|
|
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.)
|
|
(Address of principal executive offices) |
|
(Zip Code) |
(
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
|
|
|
The |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
|
|
|
|
|
||||
|
|
|
☒ |
|
Smaller reporting company |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Emerging growth company |
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes
On May 8, 2023, there were
FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q, including the sections titled “Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, contains forward-looking statements that involve risks and uncertainties. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. All statements, other than statements of historical facts contained herein, regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these identifying words. These forward-looking statements include, but are not limited to, statements about:
|
|
• |
the implementation of our business strategies, including our ability to pursue development pathways and regulatory strategies for MT-6402, MT-8421, MT-0169 and other engineered toxin body (“ETB”) biologic candidates; |
|
|
• |
our ability to resolve the partial clinical hold placed on our clinical studies of MT-0169 and to potentially resume enrollment in our MT-0169 studies; |
|
|
• |
our utilization of a de-immunized ETB scaffold that has been designed to reduce or eliminate the propensity for innate immunity, including capillary leak syndrome (“CLS”), via de-immunization of the Shiga-like Toxin A subunit (“SLTA”) as well as chemistry, manufacturing, and controls (“CMC”) improvements; |
|
|
• |
the timing and our ability to advance the development of our drug or biologic candidates; |
|
|
• |
our plans to pursue discussions with regulatory authorities, and the anticipated timing, scope and outcome of related regulatory actions or guidance; |
|
|
• |
our ability to establish and maintain potential new partnering or collaboration arrangements for the development and commercialization of ETB biologic candidates; |
|
|
• |
our ability to obtain the benefits we anticipate from partnering, collaboration, or supply agreements that we may enter into; |
|
|
• |
our financial condition, including our ability to obtain the funding necessary to advance the development of our drug or biologic candidates; |
|
|
• |
the anticipated progress of our drug or biologic candidate development programs, including whether our ongoing and potential future clinical trials will achieve clinically relevant results; |
|
|
• |
our ability to generate data and conduct analyses to support the regulatory approval of our drug or biologic candidates; |
|
|
• |
our ability to establish and maintain intellectual property rights for our drug or biologic candidates; |
|
|
• |
whether any drug or biologic candidates that we are able to commercialize are safer or more effective than other marketed products, treatments or therapies; |
|
|
• |
our ability to discover and develop additional drug or biologic candidates suitable for clinical testing; |
|
|
• |
our ability to identify, in-license or otherwise acquire additional drug or biologic candidates and development programs; |
|
|
• |
our anticipated research and development activities and projected expenditures; |
|
|
• |
our ability to complete preclinical and clinical testing successfully for new drug or biologic candidates that we may develop or license; |
|
|
• |
our ability to have manufactured active pharmaceutical ingredient (“API”) and drug or biologic product that meet required release and stability specifications; |
|
|
• |
our ability to have manufactured sufficient supplies of drug product for clinical testing and commercialization; |
|
|
• |
our ability to obtain licenses to any necessary third-party intellectual property; |
|
|
• |
our anticipated use of proceeds from any financing activities; |
|
|
• |
the expected cost savings from our recently announced strategic restructuring; |
|
|
• |
the extent to which global economic and political developments, including the indirect and/or long-term impact of the COVID-19 pandemic and inflation, will affect our business operations, clinical trials, or financial condition; |
|
|
• |
the impact of laws and regulations; |
|
|
• |
our projected financial performance, the future possibility of a strategic transaction or financing alternative, and compliance with existing debt covenants; |
|
|
• |
the sufficiency of our cash resources; and |
|
|
|
|
• |
other risks and uncertainties, including those listed under Part II, Item 1A, “Risk Factors”. |
Any forward-looking statements in this Quarterly Report on Form 10-Q reflect our current views with respect to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under Part II, Item 1A, “Risk Factors” and elsewhere in this Quarterly Report on Form 10-Q. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
This Quarterly Report on Form 10-Q also contains estimates, projections and other information concerning our industry, our business, and the markets for certain diseases, including data regarding the incidence and prevalence of certain medical conditions. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources.
As used in this Quarterly Report on Form 10-Q, unless otherwise stated or the context otherwise indicates, references to “Molecular,” the “Company,” “we,” “our,” “us” or similar terms refer to Molecular Templates, Inc., and our wholly-owned subsidiary.
Molecular Templates, Inc.
TABLE OF CONTENTS
|
|
|
|
Page |
|
|
PART I. |
|
|
4 |
|
|
Item 1. |
|
|
4 |
|
|
|
|
|
4 |
|
|
|
|
|
5 |
|
|
|
|
Condensed Consolidated Statements of Comprehensive Income (Loss) (Unaudited) |
|
6 |
|
|
|
Condensed Consolidated Statements of Stockholders’ (Deficit) Equity (Unaudited) |
|
7 |
|
|
|
|
8 |
|
|
|
|
Notes to Condensed Consolidated Financial Statements (Unaudited) |
|
9 |
|
Item 2. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
19 |
|
Item 3. |
|
|
28 |
|
|
Item 4. |
|
|
28 |
|
|
PART II. |
|
|
29 |
|
|
Item 1 |
|
|
29 |
|
|
Item 1A. |
|
|
29 |
|
|
Item 2. |
|
|
71 |
|
|
Item 3. |
|
|
71 |
|
|
Item 4. |
|
|
71 |
|
|
Item 5. |
|
|
71 |
|
|
Item 6. |
|
|
72 |
|
|
|
73 |
|||
3
PART I. FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS
Molecular Templates, Inc.
CONDENSED CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
|
|
|
March 31, 2023(unaudited) |
|
|
December 31, 2022 |
|
||
|
ASSETS |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
|
|
|
$ |
|
|
|
Marketable securities, current |
|
|
|
|
|
|
|
|
|
Prepaid expenses |
|
|
|
|
|
|
|
|
|
Grants revenue receivable |
|
|
|
|
|
|
|
|
|
Other current assets |
|
|
|
|
|
|
|
|
|
Total current assets |
|
|
|
|
|
|
|
|
|
Operating lease right-of-use assets |
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
|
|
|
|
|
|
|
|
Other assets |
|
|
|
|
|
|
|
|
|
Total assets |
|
$ |
|
|
|
$ |
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
|
|
|
$ |
|
|
|
Accrued liabilities |
|
|
|
|
|
|
|
|
|
Deferred revenue, current |
|
|
|
|
|
|
|
|
|
Other current liabilities |
|
|
|
|
|
|
|
|
|
Total current liabilities |
|
|
|
|
|
|
|
|
|
Deferred revenue, long-term |
|
|
|
|
|
|
|
|
|
Long-term debt, net of current portion |
|
|
|
|
|
|
|
|
|
Operating lease liabilities |
|
|
|
|
|
|
|
|
|
Other liabilities |
|
|
|
|
|
|
|
|
|
Total liabilities |
|
|
|
|
|
|
|
|
|
Commitments and contingencies (Note 10) |
|
|
|
|
|
|
|
|
|
Stockholders’ deficit |
|
|
|
|
|
|
|
|
|
Preferred stock, $ |
|
|
|
|
|
|
|
|
|
Authorized: December 31, 2022; issued and outstanding: March 31, 2023 and December 31, 2022 |
|
|
|
|
|
|
|
|
|
Common stock, $ |
|
|
|
|
|
|
|
|
|
Authorized: December 31, 2022; issued and outstanding: March 31, 2023 and December 31, 2022 |
|
|
|
|
|
|
|
|
|
Additional paid-in capital |
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income/(loss) |
|
|
|
|
|
|
( |
) |
|
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
|
Total stockholders’ deficit |
|
|
( |
) |
|
|
( |
) |
|
Total liabilities and stockholders’ deficit |
|
$ |
|
|
|
$ |
|
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
4
Molecular Templates, Inc.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share and per share data)
(unaudited)
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2023 |
|
|
2022 |
|
||
|
Research and development revenue |
|
$ |
|
|
|
$ |
|
|
|
Grant revenue |
|
|
|
|
|
|
|
|
|
Total revenue |
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Research and development |
|
|
|
|
|
|
|
|
|
General and administrative |
|
|
|
|
|
|
|
|
|
Total operating expenses |
|
|
|
|
|
|
|
|
|
Income/(loss) from operations |
|
|
|
|
|
|
( |
) |
|
Interest and other income, net |
|
|
|
|
|
|
|
|
|
Interest and other expense, net |
|
|
( |
) |
|
|
( |
) |
|
Net income/(loss) |
|
$ |
|
|
|
$ |
( |
) |
|
Net income/(loss) per share attributable to common shareholders: |
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
$ |
|
|
|
$ |
( |
) |
|
Weighted average number of shares used in net income/(loss) per share calculations: |
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
5
Molecular Templates, Inc.
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME/(LOSS)
(in thousands, except share and per share data)
(unaudited)
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2023 |
|
|
2022 |
|
||
|
Net income/(loss) |
|
$ |
|
|
|
$ |
( |
) |
|
Other comprehensive income/(loss): |
|
|
|
|
|
|
|
|
|
Unrealized gain/(loss) on available-for-sale securities |
|
|
|
|
|
|
( |
) |
|
Comprehensive income/(loss) |
|
$ |
|
|
|
$ |
( |
) |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
6
MOLECULAR TEMPLATES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ (DEFICIT) EQUITY
(in thousands, except share data)
(unaudited)
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2023 |
|
|
2022 |
|
||
|
Total Stockholders' (Deficit) Equity, beginning balances |
|
$ |
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred Stock: |
|
|
|
|
|
|
|
|
|
Beginning balance |
|
|
|
|
|
|
|
|
|
Issuance of preferred stock |
|
|
— |
|
|
|
— |
|
|
Ending balance |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock: |
|
|
|
|
|
|
|
|
|
Beginning balance |
|
|
|
|
|
|
|
|
|
Issuance of common stock pursuant to public offering |
|
|
|
|
|
|
|
|
|
Ending balance |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional Paid-In Capital |
|
|
|
|||||
|
Beginning balance |
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
|
|
|
|
|
|
|
|
Ending balance |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated Other Comprehensive Income/(Loss): |
|
|
|
|
|
|
|
|
|
Beginning balance |
|
|
( |
) |
|
|
( |
) |
|
Other comprehensive income/(loss) |
|
|
|
|
|
|
( |
) |
|
Ending balance |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
Accumulated deficit: |
|
|
|
|
|
|
|
|
|
Beginning balance |
|
|
( |
) |
|
|
( |
) |
|
Net income/(loss) |
|
|
|
|
|
|
( |
) |
|
Ending balance |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
Total Stockholders' (Deficit) Equity |
|
$ |
( |
) |
|
$ |
|
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
7
Molecular Templates, Inc.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
(unaudited)
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2023 |
|
|
2022 |
|
||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
Net income/(loss) |
|
$ |
|
|
|
$ |
( |
) |
|
Adjustments to reconcile net income/(loss) to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation, amortization and other |
|
|
|
|
|
|
|
|
|
Stock-based compensation expense |
|
|
|
|
|
|
|
|
|
Interest accrued on long-term debt |
|
|
|
|
|
|
|
|
|
Amortization of debt discount and accretion related to debt |
|
|
|
|
|
|
|
|
|
Accretion of asset retirement obligations |
|
|
|
|
|
|
|
|
|
Loss on disposal of property and equipment |
|
|
|
|
|
|
|
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
Prepaid expenses |
|
|
|
|
|
|
|
|
|
Grants revenue receivable |
|
|
( |
) |
|
|
|
|
|
Other assets |
|
|
( |
) |
|
|
( |
) |
|
Operating lease right-of-use assets and liabilities |
|
|
( |
) |
|
|
( |
) |
|
Accounts payable |
|
|
|
|
|
|
( |
) |
|
Accrued liabilities |
|
|
( |
) |
|
|
( |
) |
|
Deferred revenue |
|
|
( |
) |
|
|
( |
) |
|
Net cash used in operating activities |
|
|
( |
) |
|
|
( |
) |
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
|
( |
) |
|
|
( |
) |
|
Purchase of marketable securities |
|
|
( |
) |
|
|
( |
) |
|
Sales of marketable securities |
|
|
|
|
|
|
|
|
|
Net cash provided by investing activities |
|
|
|
|
|
|
|
|
|
Net increase/(decrease) in cash, cash equivalents, and restricted cash |
|
|
|
|
|
|
( |
) |
|
Cash, cash equivalents and restricted cash, beginning of period |
|
|
|
|
|
|
|
|
|
Cash, cash equivalents and restricted cash, end of period |
|
$ |
|
|
|
$ |
|
|
|
Reconciliation of cash, cash equivalents and restricted cash |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
|
|
|
$ |
|
|
|
Restricted cash included in other assets |
|
|
|
|
|
|
|
|
|
Total cash, cash equivalents and restricted cash |
|
$ |
|
|
|
$ |
|
|
|
Supplemental Cash Flow Information |
|
|
|
|
|
|
|
|
|
Cash paid for interest |
|
$ |
|
|
|
$ |
|
|
|
Non-Cash Investing Activities |
|
|
|
|
|
|
|
|
|
Fixed asset additions in accounts payable and accrued expenses |
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
8
Molecular Templates, Inc.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 — ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Nature of the Business
Molecular Templates, Inc. (the “Company”) is a clinical stage biopharmaceutical company formed in 2001, with a biologic therapeutic platform for the development of novel targeted therapeutics for cancer, headquartered in Austin, Texas. The Company’s focus is on the research and development of therapeutic compounds for a variety of cancers. The Company operates its business as a single segment, as defined by U.S. generally accepted accounting principles (“U.S. GAAP”).
Basis of Presentation
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America and include the accounts of the Company and its wholly owned subsidiary and reflect the elimination of intercompany accounts and transactions.
The preparation of condensed consolidated financial statements requires management to make estimates and assumptions that affect the recorded amounts reported therein. A change in facts or circumstances surrounding the estimates could result in a change to estimates and impact future operating results. Certain accounts in the prior financial statements have been reclassified for comparative purposes to conform to the presentation in the current financial statements. These reclassifications have no material effect on previously reported financials.
The unaudited condensed consolidated financial statements and related disclosures have been prepared with the presumption that users of the interim unaudited condensed consolidated financial statements have read or have access to the audited consolidated financial statements for the preceding fiscal year. Accordingly, these unaudited condensed consolidated financial statements should be read in conjunction with the audited consolidated financial statements and notes thereto for the year ended December 31, 2022 included in the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission (the “SEC”) on March 30, 2023.
Going Concern
The Company has adopted as required the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (ASC) Topic 205-40, Presentation of Financial Statements - Going Concern, which requires that management contemplate the realization of assets and liquidation of liabilities in the normal course of business, and evaluate whether there are relevant conditions and events that in aggregate raise substantial doubt about the entity’s ability to continue as a going concern and to meet its obligations as they become due within one year after the date that the financial statements are issued. Under this standard, management’s assessment shall not take into consideration the potential mitigating effects of management’s plans that have not been fully implemented as of the date the financial statements are issued.
There is substantial doubt about the Company’s ability to continue as a going concern as of the date of this Quarterly Report on Form 10-Q. This substantial doubt relates to the Company’s future compliance with the financial covenant in its Loan and Security Agreement with K2 HealthVentures LLC (the “K2 Loan and Security Agreement”), which requires the Company to certify monthly that it has cash, cash equivalents and marketable securities of at least five times the Company’s cash monthly burn as defined in the agreement (the “Financial Covenant”), as well as the Company’s ability to avoid triggering an event of default related to its solvency (an “Insolvency Event of Default”) under the K2 Loan and Security Agreement. Currently, based on anticipated cost-savings from the restructuring, discussed in Note 12 “Restructuring Related Expenses,” the Company anticipates continued compliance with the Financial Covenant and anticipates the ability to avoid triggering an event of default related to an Insolvency Event of Default late into the third quarter of 2023. However, the Company will require additional funding in order to meet its covenant requirements and ongoing operations. If the Company cannot raise additional capital to maintain its compliance thereafter or negotiate an amendment to the Financial Covenant or the Insolvency Events of Default, then the Company will be in default of the K2 Loan and Security Agreement and the repayment of the Company’s indebtedness may be accelerated in full by K2 HealthVentures LLC. The Company has not yet established an ongoing source of revenues sufficient to cover its operating costs and to provide sufficient certainty that it will continue as a going concern. As of March 31, 2023, the Company had an accumulated deficit of $
At March 31, 2023, the Company had cash, cash equivalents, and marketable securities of $
9
additional capital and continue as a going concern, it might have to liquidate its assets, and the values it receives for its assets in liquidation or dissolution could be significantly lower than the values reflected in its financial statements.
These financial statements do not give effect to any adjustments which will be necessary should the Company be unable to continue as a going concern and therefore be required to realize its assets and discharge its liabilities in other than the normal course of business and at amounts different from those reflected in the accompanying financial statements.
Significant Accounting Policies
There have been no material changes to the Company’s significant accounting policies during the three months ended March 31, 2023, as compared to the significant accounting policies disclosed in Note 1, “Summary of Significant Accounting Policies”, to the consolidated financial statements in the Company’s Annual Report on Form 10-K for the year ended December 31, 2022.
Cash and Cash Equivalents
The Company considers temporary investments having original maturities of three months or less from date of purchase to be cash equivalents. Restricted cash is recorded in other assets, based on when the restrictions expire. Other assets include $
Fair Value Measurement
The Company accounts for its marketable securities in accordance with ASC 820 “Fair Value Measurements and Disclosures.” ASC 820 defines fair value, establishes a framework for measuring fair value in U.S. GAAP, and expands disclosures about fair value measurements. ASC 820 defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The standard describes three levels of inputs that may be used to measure fair value:
Level 1—Quoted prices in active markets for identical assets or liabilities.
Level 2—Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The Company utilizes the market approach to measure fair value for its financial assets and liabilities. The market approach uses prices and other relevant information generated by market transactions involving identical or comparable assets or liabilities. For Level 2 securities that have market prices from multiples sources, a “consensus price” or a weighted average price for each of these securities can be derived from a distribution-curve-based algorithm which includes market prices obtained from a variety of industrial standard data providers (e.g. Bloomberg), security master files from large financial institutions, and other third-party sources. Level 2 securities with short maturities and infrequent secondary market trades are typically priced using mathematical calculations adjusted for observable inputs when available.
Concentration of Credit Risk and Other Risks and Uncertainties
Financial instruments that potentially subject the Company to concentrations of risk consist principally of cash and cash equivalents, investments, long term debt and accounts receivable.
The Company’s cash and cash equivalents are with two major financial institutions in the United States.
The Company performs an ongoing credit evaluation of its strategic partners’ financial conditions and generally does not require collateral to secure accounts receivable from its strategic partners. At March 31, 2023, the Company’s exposure to credit risk associated with non-payment will be affected principally by conditions or occurrences within Bristol Myers Squibb Company (“Bristol Myers Squibb”). In past years, the Company’s exposure to credit risk associated with non-payment were also affected principally by conditions or occurrences within Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd. (“Takeda”). Takeda accounted for approximately
10
Drug or biologic candidates developed by the Company may require approvals or clearances from the U.S. Food and Drug Administration (“FDA”) or international regulatory agencies prior to commercial sales. There can be no assurance that the Company’s drug or biologic candidates will receive any of the required approvals or clearances. If the Company were to be denied approval or clearance or any such approval or clearance were to be delayed, it would have a material adverse impact on the Company.
Recently Issued Accounting Pronouncements
In August 2020, the FASB issued ASU No. 2020-06, Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (Subtopic 470-20: Debt with Conversion and Other Options and Subtopic 815-40: Derivatives and Hedging - Contracts in Entity’s Own Equity). The new guidance simplifies accounting for convertible instruments by removing major separation models, removes certain settlement conditions that are required for equity contracts to qualify for the derivative scope exception, and it also simplifies the diluted earnings per share calculation in certain areas. The amendment is effective for the Company for fiscal years beginning after December 15, 2023. The Company does not expect this to have a material impact since there are no material convertible instruments at this time.
NOTE 2 — NET INCOME/(LOSS) PER COMMON SHARE
Basic net income/(loss) per common share is computed by dividing net income/(loss) by the weighted-average number of common shares outstanding during the period utilizing the two-class method. Preferred Stock Shareholders participate equally with Common Stock Shareholders in earnings, but do not participate in losses, and are excluded from the basic net income/(loss) calculation. Diluted net income/(loss) per share is computed by giving effect to all potential dilutive common shares, including outstanding options, warrants and convertible preferred stock. More specifically, at March 31, 2023 and March 31, 2022, stock options, warrants and, if converted, preferred stock totaling approximately
NOTE 3 — RESEARCH AND DEVELOPMENT AGREEMENTS
Disaggregated Research and Development Revenue
Research and development revenue is attributable to regions based on the location of each of the Company’s collaboration partner's parent company headquarters. Research and development revenues disaggregated by location were as follows (in thousands):
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2023 |
|
|
2022 |
|
||
|
United States |
|
$ |
|
|
|
$ |
|
|
|
Japan |
|
|
|
|
|
|
|
|
|
Total research and development revenue |
|
$ |
|
|
|
$ |
|
|
Collaboration Agreements
Bristol Myers Squibb Collaboration Agreement
In February 2021, the Company, entered into a Collaboration Agreement (the “BMS Collaboration Agreement”), as amended, with Bristol Myers Squibb to perform strategic research collaboration leveraging the Company’s ETB technology platform to discover and develop novel products containing ETBs directed to multiple targets.
Pursuant to the terms of the BMS Collaboration Agreement, the Company granted Bristol Myers Squibb a series of exclusive options to obtain one or more exclusive licenses under the Company’s intellectual property to exploit products containing ETBs directed against certain targets designated by Bristol Myers Squibb.
Bristol Myers Squibb paid the Company an upfront payment of $
The Company will be responsible for conducting the research activities through the designation, if any, of one or more development candidates. Upon the exercise of its option for a development candidate, Bristol Myers Squibb will be responsible for all development, manufacturing, regulatory and commercialization activities with respect to that development candidate.
11
Unless earlier terminated, the BMS Collaboration Agreement will expire (i) on a country-by-country basis and licensed product-by-licensed product basis, on the date of expiration of the royalty payment obligations under the BMS Collaboration Agreement with respect to such licensed product in such country and (ii) in its entirety upon the earlier of (a) the expiration of the royalty payment obligations under the BMS Collaboration Agreement with respect to all licensed products in all countries or (b) upon Bristol Myers Squibb’s decision not to exercise any option on or prior to the applicable option deadlines. Bristol Myers Squibb has the right to terminate the BMS Collaboration Agreement for convenience upon prior written notice to the Company. Either party has the right to terminate the BMS Collaboration Agreement (a) for the insolvency of the other party or (b) subject to specified cure periods, in the event of the other party’s uncured material breach. The Company has the right upon prior written notice to terminate the BMS Collaboration Agreement in the event that Bristol Myers Squibb or any of its affiliates asserts a challenge against the Company’s patents.
The Company identified multiple performance obligations at the inception of the BMS Collaboration Agreement consisting of research and development services and material rights related to additional developmental targets. The transaction price of $
The Company recognizes revenue for research and development services under the BMS Collaboration Agreement using a cost-based input measure. In applying the cost-based input method of revenue recognition, the Company will use actual costs incurred relative to budgeted costs expected to be incurred. These costs consist primarily of internal employee efforts and third-party contract costs. Revenue is recognized based on actual costs incurred as a percentage of total budgeted costs as the Company completes its performance obligation over the estimated service period.
For the three months ended March 31, 2023 and March 31, 2022, the Company recognized $
Takeda Multi-Target Agreement
In June 2017, the Company entered into a Multi-Target Collaboration and License Agreement with Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda (the “Takeda Multi-Target Agreement”), in which the Company agreed to collaborate with Takeda to identify and generate ETBs, against two targets designated by Takeda. In March 2022, following the Company’s request to bring the agreement to an end, the Company and Takeda mutually agreed to terminate the Takeda Multi-Target Agreement. As a result of the termination, the Company regained full rights to pursue the targets worked on under the Takeda Multi-Target Agreement. There are no ongoing activities or economic obligations in connection with the Takeda Multi-Target Agreement.
For the three months ended March 31, 2023, the Company did
Grant Agreements
In September 2018, the Company entered into a Cancer Research Agreement (the “CD38 CPRIT Agreement”) with the Cancer Prevention and Research Institute of Texas (“CPRIT”) which was extended in September 2022, under which CPRIT awarded a $
For the three months ended March 31, 2023 and March 31, 2022, the Company recognized grant revenue under this award of $
12
NOTE 4 — RELATED PARTY TRANSACTIONS
Takeda
In connection with the Takeda Multi-Target Agreement described in Note 3 “Research and Development Collaboration Agreements,” Takeda became a related party, following the Takeda Stock Purchase Agreement described in Note 11 “Stockholders’ Equity,” of the Company’s previously filed Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on March 30, 2023. In August 2021, Takeda ceased to be a related party after a sale of the above-mentioned shares.
NOTE 5 —MARKETABLE SECURITIES AND FAIR VALUE MEASUREMENTS
The following table sets forth the Company’s financial assets (cash equivalents and marketable securities) at fair value on a recurring basis (in thousands):
|
|
|
|
|
|
Basis of Fair Value Measurements |
|
|||||||||
|
|
March 31, 2023 |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
||||
|
Money market funds |
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Commercial paper |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
United States Treasury Bills |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate Bonds |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Amounts included in: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Marketable securities, current |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cash equivalents and marketable securities |
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basis of Fair Value Measurements |
|
|||||||||
|
|
December 31, 2022 |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
||||
|
Money market funds |
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Commercial paper |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
United States Treasury Bills |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Amounts included in: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Marketable securities, current |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cash equivalents and marketable securities |
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company invests in highly-liquid, investment-grade securities. The following is a summary of the Company’s available-for-sale securities (in thousands):
|
|
March 31, 2023 |
|
|||||||||||||
|
|
Cost Basis |
|
|
Unrealized Gain |
|
|
Unrealized Loss |
|
|
Fair Value |
|
||||
|
Cash equivalents - money market funds, commercial paper and corporate bonds |
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
|
Marketable securities, current - commercial paper |
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
|
December 31, 2022 |
|
|||||||||||||
|
|
Cost Basis |
|
|
Unrealized Gain |
|
|
Unrealized Loss |
|
|
Fair Value |
|
||||
|
Cash equivalents - money market funds, commercial paper and corporate bonds |
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Marketable securities, current - commercial paper, Treasury bills |
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
13
At both March 31, 2023 and December 31, 2022, all the Company’s available-for-sale investments were due in one year or less.
The Company received
NOTE 6 — BALANCE SHEET COMPONENTS
Accrued liabilities consisted of the following (in thousands):
|
|
|
March 31, 2023 |
|
|
December 31, 2022 |
|
||
|
Accrued liabilities: |
|
|
|
|
|
|
|
|
|
General and administrative |
|
$ |
|
|
|
$ |
|
|
|
Clinical trial related costs |
|
|
|
|
|
|
|
|
|
Non-clinical research and manufacturing operations |
|
|
|
|
|
|
|
|
|
Payroll related |
|
|
|
|
|
|
|
|
|
Other accrued expenses |
|
|
|
|
|
|
|
|
|
Total Accrued liabilities |
|
$ |
|
|
|
$ |
|
|
NOTE 7—PROPERTY AND EQUIPMENT
Property and equipment consists of the following (in thousands):
|
|
|
March 31, |
|
|
December 31, |
|
||
|
|
|
2023 |
|
|
2022 |
|
||
|
Laboratory equipment |
|
$ |
|
|
|
$ |
|
|
|
Leasehold improvements |
|
|
|
|
|
|
|
|
|
Furniture and fixtures |
|
|
|
|
|
|
|
|
|
Computer and equipment |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Less: Accumulated depreciation |
|
|
( |
) |
|
|
( |
) |
|
Total property and equipment, net |
|
$ |
|
|
|
$ |
|
|
Depreciation expense was $
In connection with the continued expansion of the Company’s facilities, at both March 31, 2023 and December 31, 2022, the Company had net Asset Retirement Obligation (ARO) assets totaling $
NOTE 8 — BORROWING ARRANGEMENTS
K2 HealthVentures Loan and Security Agreement
In May 2020, the Company entered into the K2 Loan and Security Agreement in the amount of $
The K2 Loan and Security Agreement includes both financial and non-financial covenants including the Financial Covenant as well as solvency requirements, the breach of which would trigger an event of default.
As of both March 31, 2023 and December 31, 2022, the K2 Loan principal balance was $
14
As of March 31, 2023 and December 31, 2022, the carrying value of long-term debt was $
Future required principal and final payments on the K2 Loan were as follows at March 31, 2023 ($ in thousands):
|
2023 |
|
$ |
|
|
|
2024 |
|
|
|
|
|
Total Principal Amounts |
|
|
|
|
|
Final Fee Due at Maturity |
|
|
|
|
|
Unamortized discount, deferred costs and final fee |
|
|
( |
) |
|
Total Long-Term Debt, net |
|
$ |
|
|
NOTE 9 – LEASES
The Company has operating leases for administrative offices and research and development facilities, and certain finance leases for equipment. The operating leases have remaining terms of less than
In July 2022, the Company exercised its option to extend the term for its lease of its principal executive office at 9301 Amberglen Blvd, Building J, Austin TX 78729 (the “Property”) for an additional term beginning
In October 2022, the Company entered into that certain Fourth Amendment to Lease between the Company and NW Austin Office Partners LLC (the “Lease Amendment”) which amended the Lease Agreement to document the exercise of the Company’s option to extend the term of its lease of the Property for an additional term beginning
The following table summarizes the components of lease expense for the three months ended March 31, 2023 and March 31, 2022 and (in thousands):
|
|
Three Months Ended March 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
|
Operating leases |
|
|
|
|
|
|
|
|
Operating lease expense |
$ |
|
|
|
$ |
|
|
|
Variable lease expense |
|
|
|
|
|
|
|
|
Total operating lease expense |
$ |
|
|
|
$ |
|
|
15
The following table summarizes the balance sheet classification of leases at March 31, 2023 (in thousands):
|
|
March 31, |
|
|
|
|
2023 |
|
|
|
Operating leases |
|
|
|
|
Operating lease right-of-use assets |
$ |
|
|
|
|
|
|
|
|
Operating lease liabilities, current1 |
$ |
|
|
|
Operating lease liabilities, non-current |
|
|
|
|
Total operating lease liabilities |
$ |
|
|
|
|
1. |
Included in other current liabilities. |
The following table presents other information on leases as of March 31, 2023 and December 31, 2022:
|
|
|
March 31, |
|
December 31, |
|
|
|
2023 |
|
2022 |
|
Weighted average remaining lease term, operating leases |
|
|
|
|
|
Weighted average discount rate, operating leases |
|
|
|
|
Maturities of lease liabilities were as follows as of March 31, 2023 (in thousands):
|
|
|
Operating Leases |
|
|
|
2023 (remaining) |
|
$ |
|
|
|
2024 |
|
|
|
|
|
2025 |
|
|
|
|
|
2026 |
|
|
|
|
|
2027 |
|
|
|
|
|
Thereafter |
|
|
|
|
|
Total lease payments |
|
$ |
|
|
|
Less: |
|
|
|
|
|
Imputed interest |
|
|
( |
) |
|
Total lease liabilities |
|
$ |
|
|
Supplemental cash flow information related to the Company’s leases were as follows (in thousands):
|
|
|
Three Months Ended March 31, |
|
|
|
|
|
2023 |
|
|
|
Cash paid for amounts included in the measurement of lease liabilities: |
|
|
|
|
|
Operating cash flows operating leases |
|
$ |
|
|
16
NOTE 10 — CONTRACTUAL COMMITMENTS
The Company has entered into project work orders for each of its clinical trials with clinical research organizations (each being a “CRO”) and related laboratory vendors. Under the terms of these agreements, the Company is required to pay certain upfront fees for direct services costs. Based on the particular agreement some of the fees may be for services yet to be rendered and are reflected as a current prepaid asset and have an unamortized balance of approximately
The Company has entered into estimated purchase obligations, which include signed orders for capital equipment. These estimated purchase obligations total in range from $
NOTE 11 — STOCK-BASED COMPENSATION
Stock-based compensation expense, which consists of the compensation cost for employee stock options and the value of options issued to non-employees for services rendered, was allocated to research and development and general and administrative in the consolidated statements of operations as follows (in thousands):
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2023 |
|
|
2022 |
|
||
|
Research and development |
|
$ |
|
|
|
$ |
|
|
|
General and administrative |
|
|
|
|
|
|
|
|
|
Total stock-based compensation |
|
$ |
|
|
|
$ |
|
|
At March 31, 2023, the total unrecognized compensation cost related to unvested stock-based awards granted to employees under the Company’s equity incentive plans was approximately $
Valuation Assumptions
The Company estimated the fair value of stock options granted using the Black-Scholes option-pricing formula and a single option award approach. This fair value is being amortized ratably over the requisite service periods of the awards, which is generally the vesting period.
The fair value of employee stock options was estimated using the following weighted-average assumptions:
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2023 |
|
|
2022 |
|
||
|
Employee Stock Options: |
|
|
|
|
|
|
|
|
|
Risk-free interest rate |
|
|
|
% |
|
|
|
% |
|
Expected term (in years) |
|
|
|
|
|
|
|
|
|
Dividend yield |
|
|
|
|
|
|
|
|
|
Volatility |
|
|
|
% |
|
|
|
% |
|
Weighted-average fair value of stock options granted |
|
$ |
|
|
|
$ |
|
|
Equity Incentive Plans
These plans consist of the 2018 Equity Incentive Plan, the 2014 Equity Incentive Plan, as amended; the 2004 Amended and Restated Equity Incentive Plan; and the Amended and Restated 2004 Employee Stock Purchase Plan. As of May 31, 2018, the 2014 Equity Incentive Plan; and the 2004 Amended and Restated Equity Incentive Plan were terminated, and no further shares will be granted from those plans.
17
The following table summarizes stock option activity under the Company’s equity incentive plans:
|
|
|
Outstanding Options Number of Shares |
|
|
Weighted Average Exercise Price |
|
|
Weighted Average Remaining Contractual Term |
|
|
Aggregate Intrinsic Value (in millions): |
|
||||
|
Balances, December 31, 2021 |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
$ |
|
|
|
Granted |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
Cancelled |
|
|
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
Balances, December 31, 2022 |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
$ |
|
|
|
Granted |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
Cancelled |
|
|
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
Balances, March 31, 2023 |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vested and expected to vest, March 31, 2023 |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
$ |
|
|
|
Exercisable at March 31, 2023 |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
$ |
|
|
The total intrinsic value of stock options exercised during the three months ended both March 31, 2023 and 2022, was
Cash received from stock option exercises was
NOTE 12 – RESTRUCTURING RELATED EXPENSES
On March 29, 2023, the Company implemented a strategic reprioritization and corresponding reduction in workforce, designed to focus on the clinical development programs for MT-6402, MT-8421 and MT-0169, and preclinical activities related to the Company’s collaboration with Bristol Myers Squibb (the “Restructuring”). The Restructuring reduced the Company’s workforce by approximately
The following table summarizes the activity for the three months ended March 31, 2023 for expenses related to the restructuring accruals, which are included in Accrued liabilities in the Company’s Condensed Consolidated Balance Sheets as of March 31, 2023 (in thousands):
|
Balance, December 31, 2022 |
|
$ |
|
|
|
Restructuring expenses |
|
|
|
|
|
Balance, March 31, 2023 |
|
$ |
|
|
18
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with the unaudited financial information and the notes thereto included appearing elsewhere in this Quarterly Report on Form 10-Q, and the audited financial information and the notes thereto included in our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on March 30, 2023. Some of the information contained in this discussion and analysis or set forth elsewhere in this Quarterly Report on Form 10-Q, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this Quarterly Report on Form 10-Q, our actual results could differ materially from the results described in, or implied by, the forward-looking statements contained in the following discussion and analysis.
Overview
Molecular Templates is a clinical-stage biopharmaceutical company focused on the discovery and development of targeted biologic therapeutics. Our proprietary drug platform technology, known as engineered toxin bodies (“ETBs”), leverages the resident biology of a genetically engineered form of Shiga-like Toxin A subunit (“SLTA”) to create novel therapies with potent and differentiated mechanisms of action (“MOA”) for cancer.
Recent Developments
Partial Clinical Hold for Phase 1 Study of MT-0169
On April 7, 2023, we announced that the U.S. Food and Drug Administration (the “FDA”) informed us that it had placed a partial clinical hold on the Phase 1 study of MT-0169 based on previously disclosed cardiac adverse events noted in two patients dosed at 50 mcg/kg that prompted the dose reduction to 5 mcg/kg in early 2022. We have provided the FDA, as requested, narratives on the two patients who experienced cardiotoxicity at 50 mcg/kg, justification for the revised dose of 5 mcg/kg, and data evaluating the clinical benefit-to-risk ratio seen with the lower doses of MT-0169. We anticipate feedback from the FDA on our responses to the clinical hold by the end of May 2023. Under the partial clinical hold, current study participants may continue treatment, but no new patients will be enrolled until the partial hold is lifted by the FDA. If the FDA does not lift the partial clinical hold in the near future or at all, our clinical development of MT-0169 will be materially and adversely delayed and impaired. There can be no assurance that our current or future clinical trials will not be subject to additional partial or full clinical holds, which could delay or impair the commencement and completion of our clinical trials and the regulatory approval of our drug or biologic candidates.
Nasdaq Hearing Process regarding Potential Delisting of Common Stock
In connection with the deficiency and delisting notices received from Nasdaq as previously disclosed, we attended a hearing before the Nasdaq Hearings Panel (the “Panel”) on April 13, 2023, where we presented our plan to regain compliance with both the bid price and stockholders’ equity requirements. On May 8, 2023, we received the Panel’s decision, which granted our request for an extension to regain compliance with both the bid price and stockholders’ equity requirements by August 28, 2023, subject to certain conditions as set forth by the Panel. We continue to work diligently to regain compliance. Our plan with respect to the bid price requirement may include seeking to affect a reverse stock split, subject to obtaining stockholder approval, and to regain compliance with the stockholders’ equity requirement by raising capital. However, there can be no assurance that we will be able to raise sufficient capital to regain compliance with the applicable listing criteria such as the stockholders’ equity requirement within the extension granted by the Panel. Further, there can be no assurance that the Panel will not reconsider the terms of this extension based upon future events, conditions or circumstances that may arise with the Company, which in the opinion of the Panel, may make continued listing on the Nasdaq Capital Market inadvisable.
Process to Explore Strategic Alternatives
We have an ongoing process to explore a range of strategic and financing alternatives to maximize shareholder value. In addition to continuing to explore available financing alternatives to maintain continued compliance with the covenants and restrictions under our Loan and Security Agreement with K2 HealthVentures LLC (“K2”) (the “K2 Loan and Security Agreement”) as described below and to lengthen our cash runway, our process will focus on identifying and evaluating any other strategic alternatives, including potentially the sale of all, or part, of our assets, or a merger. We have retained the investment bank Stifel, Nicolaus & Company, Incorporated to act as a strategic advisor for this process. There can be no assurance that this strategic review process will result in the completion of any transaction. We have not set a timetable for completion of this strategic review process, and we do not intend to comment further unless or until our Board of Directors has approved a definitive course of action, the review process is concluded, or it is determined that other disclosure is appropriate.
19
Business
ETBs use a genetically engineered version of the SLTA, a ribosome inactivating bacterial protein. In its wild-type form, Shiga-like Toxin (“SLT”) is thought to induce its own entry into a cell when proximal to the cell surface membrane, self-route to the cytosol, and enzymatically and irreversibly shut down protein synthesis via ribosome inactivation. SLTA is normally coupled to its cognate Shiga-like Toxin B subunit (“SLTB”) to target the CD77 cell surface marker, a non-internalizing glycosphingolipid. In our scaffold, a genetically engineered SLTA subunit with no cognate SLTB component is genetically fused to antibody domains or fragments specific to a target, resulting in a biologic therapeutic that can identify the particular target and specifically kill the cell. The antibody domains may be substituted with other antibody domains having different specificities to allow for the rapid development of new drugs to selected targets in cancer.
ETBs combine the specificity of an antibody with SLTA’s potent mechanism of cell destruction. Based on the disease setting, we have created ETBs that have a reduced propensity for triggering innate immunogenicity and attendant toxicities like capillary leak syndrome (“CLS”). To date, there have been no instances of CLS or other manifestations of innate immunity observed with any of our next-generation ETBs.
ETBs have relatively predictable pharmacokinetic (“PK”) profiles and can be rapidly screened for desired activity in robust cell-based and animal-model assays. Because SLTA can induce internalization against non- and poorly-internalizing receptors, the universe of targets for ETBs should be substantially larger than that seen with antibody drug conjugates (“ADCs”), which are not likely to be effective if the target does not readily internalize the ADC payload.
ETBs have a differentiated mechanism of cell kill in cancer therapeutics (the inhibition of protein synthesis via ribosome destruction), and we have preclinical and clinical data demonstrating the utility of these molecules in chemotherapy-refractory cancers. ETBs have shown good tolerability in multiple animal models as well as a generally favorable tolerability profile in our clinical studies to date. We believe the target specificity of ETBs, their ability to self-internalize, their potent and differentiated mechanism of cell kill and their tolerability profile provide opportunities for the clinical development of these agents to address multiple cancer types.
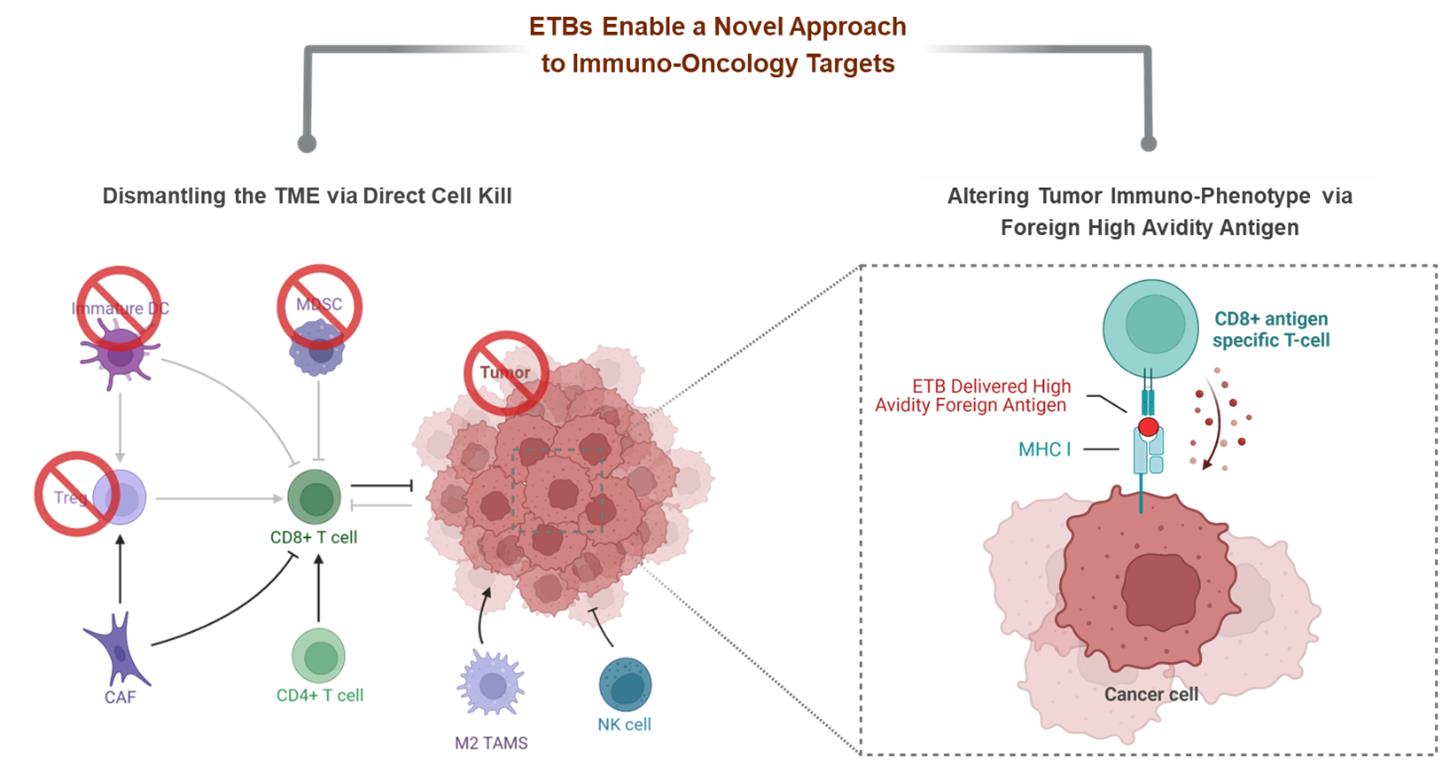
We have developed ETBs to various targets, including PD-L1, CD38, and CTLA-4. PD-L1 and CTLA-4 are key immune checkpoint pathways and are validated targets expressed in a variety of solid tumor cancers and immune cells. The differentiated mechanism of action of our ETBs allows for a novel approach to mediating anti-tumor T-cell activity against immuno-oncology targets by: (i) dismantling the tumor micro-environment (“TME”) through the depletion of immunosuppressive immune cells and (ii) delivering high avidity major histocompatibility complex-I (“MHC-I”) antigens to the tumor to directly alter the tumor’s immunophenotype. The altering of the tumor’s immunophenotype is unique and leverages the intrinsic intracellular routing properties of ETBs through a mechanism we call Antigen Seeding.

Immuno-Oncology ETBs
MT-6402 – ETB Targeting PD-L1
We filed an Investigational New Drug (“IND”) application for MT-6402, our ETB targeting PD-L1, in December 2020 and the IND was accepted in January 2021. A Phase I study of MT-6402 in relapsed/refractory patients with PD-L1 expressing tumors began in July 2021 at a starting dose of 16 mcg/kg. The Phase I study for MT-6402 is a multi-center, open-label, dose escalation and dose
20
expansion trial. Patients with confirmed PD-L1 expressing tumors or confirmed PD-L1 expression in the TME are eligible for enrollment, irrespective of HLA genotype or CMV status. Following a review of the safety data from cohort 6 (83 mcg/kg), which was well tolerated, patient enrollment in cohort 7 initiated and is continuing at a dose of 100 mcg/kg. In November 2021, MT-6402 was granted Fast Track designation for the treatment of patients with advanced NSCLC expressing PD-L1. For MT-6402, dose escalation in the Phase I study continues as planned for 2023, with one expansion for patients with high PD-L1 tumor expression (≥ 50%) and the other expansion for patients with low (1-49%) PD-L1 tumor expression.
As of May 2023, patients have been treated across six dose escalation cohorts of 16 mcg/kg, 24 mcg/kg, 32 mcg/kg, 42 mcg/kg, 63 mcg/kg and 83 mcg/kg in the MT-6402 study of patients with relapsed/refractory tumors that express PD-L1. Dose escalation continues with patients being recruited at 100 mcg/kg. We continue to observe pharmacodynamic (“PD”) effects including the depletion of PD-L1+ monocytes, MDSCs, PD-L1+ dendritic cells, as well as T cell activation.
One patient with high tumor PD-L1 expression who also had Antigen Seeding capability, demonstrated tumor regression while being dosed with MT-6402 for over 7 months. This patient, with non-small cell lung cancer (“NSCLC”), was treated in cohort 1 (16 mcg/kg) and demonstrated resolution of three osseous lesions and a reduction in uptake in the remaining lesion. This patient also experienced grade 2 cytokine release syndrome (“CRS”) consistent with T-cell activation and was dose reduced to 8 mcg/kg. This patient had evaluable-only multiple sites of bone disease that appeared to have resolved on bone scan after 3-4 months on MT-6402 with only one remaining site which showed decreased uptake.
One patient in cohort 5 (63 mcg/kg) with metastatic squamous cell nasopharynx carcinoma with disease progression after radiation therapy, chemotherapy, and pembrolizumab had a Partial Response (“PR”) (RECIST) with a 63% reduction in the index lesion after cycle 2. The PR was confirmed after cycle 4 with a 66% reduction and the patient remains on treatment and in a response in cycle 7. This patient had 2% PD-L1 expression and was not HLA-A*02, suggesting the response is due to T-cell activation through the clearance of PD-L1+ immune cells. The patient showed a >250% increase in CD8/CD4 T-cell ratios. To date, treatment-related adverse events (“AEs”) including immune related AEs have been largely restricted to grade 1 or grade 2.
MT-8421—ETB Targeting CTLA-4
We filed an IND for MT-8421, our ETB targeting CTLA-4, in February 2023 and the IND was accepted in March 2023. MT-8421, along with MT-6402, represent our unique approach to immuno-oncology based on dismantling the TME through direct cell-kill of tumor and immune cells and not only the blocking of ligand-ligand interactions seen with current antibody therapeutics. The ETB approach includes potent destruction of CTLA-4+ regulatory T cells (“Tregs”) via enzymatic ribosome destruction, and the mechanism of cell kill is independent of TME. MT-8421 preferentially destroys high CTLA-4 expressing Tregs in the TME relative to peripheral Tregs which are lower CTLA-4 expressing. We expect to initiate a first-in-human Phase 1 study by mid-year 2023.
Hematologic Malignancy Targeted ETBs
MT-0169—ETB Targeting CD38
MT-0169, our ETB targeting CD38, had its IND filed in May 2019 and was accepted in June 2019. The Phase I study for MT-0169 in relapsed/refractory multiple myeloma initiated in the fourth quarter of 2019, with the first patient dosed in February 2020. In December 2019, the FDA granted Orphan Drug Designation to MT-0169 for the treatment of multiple myeloma.
The revised protocol for the ongoing Phase I study for MT-0169 in patients with relapsed/refractory multiple myeloma opened in January 2022. The revised protocol began at the lower dose of MT-0169 at 5 mcg/kg to reduce the risk of AEs observed at the initial dose level of 50 mcg/kg and to enable patients to continue MT-0169 therapy for a longer duration that may drive tumor benefit. We opened new sites for the Phase I study and enrollment resumed in July 2022. Following a review of the safety data from cohorts 1 (5 mcg/kg) and 2 (10 mcg/kg) in which no cardiac AEs were observed, dose escalation began in cohort 3 at 15 mcg/kg. In April 2023, the FDA placed the Phase I study for MT-0169 on a partial clinical hold based on previously disclosed cardiac AEs noted in two patients dosed at 50 mcg/kg that prompted the dose reduction to 5 mcg/kg last year. Under the partial clinical hold, current study participants may continue treatment, but no new patients will be enrolled until the partial hold is lifted by the FDA. We submitted our response to the partial clinical hold to the FDA in May 2023. Of the patients treated, one patient with extramedullary IgA myeloma treated at 5 mcg/kg has had a marked reduction in IgA serum protein, conversion from immunofixation positive to negative and resolution of uptake on bone scan of skeletal lesions demonstrating a stringent Complete Response (“CR”). The patient’s disease was quad-agent refractory including CD38-targeting, proteosome inhibitor, IMiD, and a BCMA bispecific antibody. The patient continues on study in a stringent CR at cycle 9.
We expect to provide periodic updates on MT-6402, MT-8421, and MT-0169 throughout 2023.
We have built up multiple core competencies around the creation and development of ETBs. We developed the ETB technology in-house and continue to make iterative improvements in the scaffold and identify new uses of the technology. We also developed the proprietary process for manufacturing ETBs under Current Good Manufacturing Process (“cGMP”) regulatory standards and continue to make improvements to our manufacturing processes.
We have conducted multiple cGMP manufacturing runs with our compounds and believe this process is robust and could support commercial production with gross margins that are similar to those seen with antibodies.
21
Financial Operations Overview
Revenue
To date, we have not generated any revenue from product sales to customers. We do not expect to receive any revenue from any ETB candidates that we or our current or future collaboration partners develop, including MT-6402, MT-8421, and MT-0169, until we obtain regulatory approval and commercialize such biologics. Our revenue consists principally of collaboration revenue and grant revenue.
Research and Development revenue primarily relates to our collaboration agreement with Bristol Myers Squibb which is accounted for using the percentage-of-completion cost-to-cost method.
Grant revenue relates to our CPRIT grant for a CD38 ETB (MT-0169). CPRIT grant funds for MT-0169 are provided to us in arrears as cost reimbursement where revenue is recognized as allowable costs are incurred. Revenue recognized in excess of amounts collected are recorded as grant receivable. Funds received in excess of expenditures are recorded as deferred revenue.
For more information about our revenue recognition policy, please see Note 1, “Organization and Summary of Significant Accounting Policies” to our audited consolidated financial statements for the year ended December 31, 2022, included in our Annual Report on Form 10-K.
Research and Development Expenses
Research and development expenses consist principally of:
|
|
• |
salaries for research and development staff and related expenses, including stock-based compensation expenses; |
|
|
• |
costs for current good manufacturing practices (“cGMP”) manufacturing of drug substances and drug products by contract manufacturers; |
|
|
• |
fees and other costs paid to clinical trials sites and clinical research organizations, (“CROs”), in connection with the performance of clinical trials and preclinical testing; |
|
|
• |
costs for consultants and contract research; |
|
|
• |
costs of laboratory supplies and small equipment, including maintenance; and |
|
|
• |
depreciation of long-lived assets. |
Our research and development expenses may vary substantially from period to period based on the timing of our research and development activities, including the initiation and enrollment of subjects in clinical trials and manufacture of drug or biologic materials for clinical trials. We expect research and development expenses to increase as we advance the clinical development of MT-6402, MT-8421, and/or MT-0169. The successful development of our ETB candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing and costs of the efforts that will be necessary to complete the development of, or the period, if any, in which material net cash inflows may commence from any of our ETB candidates. This is due to numerous risks and uncertainties associated with developing drugs, including the uncertainty of:
|
|
• |
the scope, rate of progress and expense of our research and development activities; |
|
|
• |
clinical trials and early-stage results; |
|
|
• |
the terms and timing of regulatory approvals; and |
|
|
• |
the ability to market, commercialize and achieve market acceptance for MT-6402, MT-8421, MT-0169, or any other ETB candidate that we or our current or future collaboration partners may develop in the future. |
Any of these variables with respect to the development of MT-6402, MT-8421, MT-0169, or any other ETB candidate that we may develop could result in a significant change in the costs and timing associated with the development of such candidates. For example, if the FDA, the European Medicines Agency (“EMA”) or other regulatory authority were to require us to conduct pre-clinical and clinical studies beyond those which we currently anticipate will be required for the completion of clinical development or if we experience significant delays in enrollment in any clinical trials, we could be required to expend significant additional financial resources and time on the completion of our clinical development programs.
General and Administrative Expenses
Our general and administrative expenses consist principally of:
|
|
• |
salaries for employees other than research and development staff, including stock-based compensation expenses; |
|
|
• |
professional fees for auditors and other consulting expenses related to general and administrative activities; |
22
|
|
• |
professional fees for legal services related to the protection and maintenance of our intellectual property and regulatory compliance; |
|
|
• |
cost of facilities, communication and office expenses; |
|
|
• |
information technology services; and |
|
|
• |
depreciation of long-lived assets. |
Other Income (Expense)
Other income (expense) mainly includes interest income earned on our cash and marketable securities balances held and interest expense on our outstanding borrowings.
Results of Operations
Revenues
The table below summarizes our revenues as follows (in thousands):
|
|
|
Three Months Ended March 31, |
|
|||||||
|
|
|
2023 |
|
|
|
|
2022 |
|
||
|
Research and development revenue |
|
$ |
33,627 |
|
|
|
|
$ |
8,486 |
|
|
Grant revenue |
|
|
3,002 |
|
|
|
|
|
— |
|
|
Total revenue |
|
$ |
36,629 |
|
|
|
|
$ |
8,486 |
|
Research and Development Revenue
The increase in research and development revenue for the three months ended March 31, 2023 compared to the three months ended March 31, 2022 was primarily due to the completion of the research program for one of the collaboration’s targets and the completion of the related performance obligations under our BMS Collaboration Agreement, resulting in recognition of $25.8 million of research and development revenue.
For more information about our prior and current collaboration agreements, please see Note 3 “Research and Development Agreements” to our unaudited consolidated financial statements for the three months ended March 31, 2023, included in this Quarterly Report on Form 10-Q.
Grant Revenue
The increase in grant revenue for the three months ended March 31, 2023 compared to the three months ended March 31, 2022 was primarily due to recognizing revenue for the CD38 CPRIT Agreement grant during the period.
Operating Expenses
The table below summarizes our operating expenses as follows (in thousands):
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2023 |
|
|
2022 |
|
||
|
Research and development expenses |
|
$ |
19,042 |
|
|
$ |
21,497 |
|
|
General and administrative expenses |
|
|
5,802 |
|
|
|
7,620 |
|
|
Total operating expenses |
|
$ |
24,844 |
|
|
$ |
29,117 |
|
23
Research and Development Expenses
The table below summarizes our research and development expenses as follows (in thousands):
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2023 |
|
|
2022 |
|
||
|
Program costs |
|
$ |
5,618 |
|
|
$ |
6,493 |
|
|
Employee compensation |
|
|
8,573 |
|
|
|
11,038 |
|
|
Laboratory costs |
|
|
2,495 |
|
|
|
831 |
|
|
Other research and development costs |
|
|
2,356 |
|
|
|
3,135 |
|
|
Total research and development expenses |
|
$ |
19,042 |
|
|
$ |
21,497 |
|
Research and development (“R&D”) expenses decreased $2.5 million during the three months ended March 31, 2023 compared to the three months ended March 31, 2022 and was primarily due to a decrease in employee compensation and program costs and partially offset by increases in laboratory costs.
Program costs decreased $0.9 million during the three months ended March 31, 2023 compared to the three months ended March 31, 2022. The program costs primarily driving the decrease for the three months ended March 31, 2023 were $0.7 million for MT-8421, $0.4 million for Bristol Myers Squibb, $0.3 million for MT-5111 and $0.1 million for MT-3724, partially offset by increases of $0.5 million for MT-0169 and $0.1 million for MT-6402.
Employee compensation costs decreased by $2.5 million for the three months ended March 31, 2023 compared to the three months ended March 31, 2022 as a result of the decrease in R&D headcount.
Laboratory costs increased by $1.7 million for the three months ended March 31, 2023 compared to the three months ended March 31, 2022. The main driver of this increase is laboratory expansion costs, supplies and equipment.
General and Administrative Expenses
General and administrative expenses decreased $1.8 million during the three months ended March 31, 2023 compared to the three months ended March 31, 2022. The main driver of this decrease was primarily related to a decrease in employee compensation costs.
Nonoperating activities
The table below summarizes our nonoperating activities as follows (in thousands):
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2023 |
|
|
2022 |
|
||
|
Interest and other income, net |
|
$ |
455 |
|
|
$ |
70 |
|
|
Interest expense |
|
|
(1,395 |
) |
|
|
(1,050 |
) |
|
Total nonoperating activities |
|
$ |
(940 |
) |
|
$ |
(980 |
) |
Interest and Other Income and Interest Expense
The increase in interest and other income, net for the three months ended March 31, 2023 compared to the three months ended March 31, 2022 was primarily due to higher interest related to our marketable securities.
The increase in interest expense for the three months ended March 31, 2023 compared to the three months ended March 31, 2022 was primarily due to the increase in our interest rate related to our debt holdings.
Liquidity and Capital Resources
Sources of Funds and Liquidity
Historically, we have funded our operations by raising capital from external sources, especially through the sale of common stock and our borrowings under the K2 Loan and Security Agreement. However, we are currently facing substantial doubt about our ability to continue as a going concern, given that our continued compliance with the financial covenant in the K2 Loan and Security Agreement, which requires us to certify monthly that we have cash, cash equivalents and marketable securities of at least five times our cash monthly burn as defined in the agreement (the “Financial Covenant”), and our ability to avoid triggering an event of default related to our solvency (an “Insolvency Event of Default”) beyond the third quarter of 2023 is dependent on us raising capital before
24
such time or being successful at negotiating an amendment to the K2 Loan and Security Agreement, see “−Recent Developments− Substantial Doubt about Going Concern related to Future Debt Compliance” in our Annual Report on Form 10-K for the year ended December 31, 2022 filed with the SEC on March 30, 2023. If we cannot raise additional capital by then to maintain ourselves in compliance or negotiate an amendment to the Financial Covenant or the Insolvency Events of Default, then we will be in default of the K2 Loan and Security Agreement and the repayment of our indebtedness may be accelerated in full by K2 HealthVentures LLC. At March 31, 2023, we had cash, cash equivalents, and marketable securities of $41.7 million, including borrowings of $35.0 million under the K2 Loan and Security Agreement whose scheduled maturity date for repayment is June 1, 2024, but a default of the Financial Covenant or an Insolvency Event of Default would potentially trigger accelerated repayment. There can no assurances that we will be able to raise sufficient capital to fund ongoing operations and maintain compliance with the Financial Covenant and avoid triggering an Insolvency Event of Default beyond the third quarter of 2023 and/or be successful at negotiating an amendment to the K2 Loan and Security Agreement thereafter.
Future Funding Requirements and Liquidity
Based on our cash, cash equivalents and marketable securities as of March 31, 2023 (approximately $41.7 million) and the anticipated cost-savings of the Restructuring and other assumptions, we anticipate that we will be able to fund our planned operating expenses and capital expenditure requirements pursuant to the priorities of our strategic refocusing into the second quarter of 2024. However, the foregoing is subject to our continued compliance with the Financial Covenant in the K2 Loan and Security Agreement, which requires us to certify monthly that we have cash, cash equivalents and marketable securities of at least five times our cash monthly burn as defined in the agreement, as well as our ability to avoid triggering an Insolvency Event of Default under the K2 Loan and Security Agreement. Currently, based on anticipated cost-savings from the Restructuring, we are anticipating continued compliance with the Financial Covenant and our ability to avoid triggering an Insolvency Event of Default late into the third quarter of 2023. If we cannot raise additional capital to maintain itself in compliance thereafter or negotiate an amendment to the Financial Covenant or to the Insolvency Events of Default, then we will be in default of the K2 Loan and Security Agreement and the repayment of our indebtedness may be accelerated in full by K2 HealthVentures LLC. As of March 31, 2023, we had $35.0 million outstanding under K2 Loan and Security Agreement, whose maturity date for repayment is June 1, 2024.
Our financial statements are prepared using U.S. generally accepted accounting principles (“U.S. GAAP”) applicable to a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business. We have not yet established an ongoing source of revenues sufficient to cover our operating costs and to provide sufficient certainty that we will continue as a going concern.
Cash Flows
Comparison of Three Months Ended March 31, 2023 and 2022
The table below summarizes our cash flows for the three months ended March 31, 2023 and 2022 (in thousands):
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2023 |
|
|
2022 |
|
||
|
Net cash used in operating activities |
|
$ |
(19,361 |
) |
|
$ |
(27,696 |
) |
|
Net cash provided by investing activities |
|
|
25,953 |
|
|
|
21,285 |
|
|
Net increase/(decrease) in cash, cash equivalents, and restricted cash |
|
$ |
6,592 |
|
|
$ |
(6,411 |
) |
The decrease in net cash used in operating activities for the three months ended March 31, 2023 compared to the three months ended March 31, 2022 was primarily due to decreases in headcount and program expenses.
The increase in net cash provided by investing activities for the three months ended March 31, 2023 compared to the three months ended March 31, 2022 was primarily due to changes of investment activity of marketable securities.
Operating and Capital Expenditure Requirements
We have not achieved profitability since our inception and had an accumulated deficit of $433.9 million at March 31, 2023. We expect to continue to incur significant operating losses for the foreseeable future as we continue our research and development efforts and seek to obtain regulatory approval and commercialization of our ETB candidates.
We expect our expenses to increase substantially in connection with our ongoing development activities related to MT-6402, MT-8421, MT-0169, and our collaboration with Bristol Myers Squibb. In addition, we expect to incur additional costs associated with operating as a public company. We anticipate that our expenses will increase substantially if and as we:
|
|
• |
support the PD-L1 program and the ongoing Phase I study for MT-6402; |
25
|
|
• |
support the planned Phase I study of MT-8421; |
|
|
• |
support the ongoing Phase I study of MT-0169; |
|
|
• |
research activities through the designation of the development candidate(s) with Bristol Myers Squibb; |
|
|
• |
seek to enhance our technology platform using our antigen-seeding technology approach to immuno-oncology; |
|
|
• |
seek regulatory approvals for any ETB candidates that successfully complete clinical trials; |
|
|
• |
potentially establish a sales, marketing and distribution infrastructure and scale up manufacturing capabilities to commercialize any drugs for which we may obtain regulatory approval; |
|
|
• |
add clinical, scientific, operational, financial and management information systems and personnel, including personnel to support our product development and potential future commercialization efforts and to support our increased operations; |
|
|
• |
experience any delays or encounter any issues resulting from any of the above, including but not limited to failed studies, complex results, safety issues or other regulatory challenges; and |
|
|
• |
service long-term debt. |
Because of the numerous risks and uncertainties associated with the development of MT-6402, MT-8421, MT-0169, and our collaboration with Bristol Myers Squibb, and because the extent to which we may enter into collaborations with third parties for development of these ETB candidates is unknown, we are unable to estimate the amount of increased capital outlays and operating expenses associated with completing the research and development of our ETB candidates. Our future capital requirements for MT-6402, MT-8421, or MT-0169 will depend on many factors, including:
|
|
• |
the progress, timing and completion of pre-clinical testing and clinical trials for our current or any future ETB candidates; |
|
|
• |
the number of potential new ETB candidates we identify and decide to develop; |
|
|
• |
the costs involved in growing our organization to the size needed to allow for the research, development and potential commercialization of our current or any future ETB candidates; |
|
|
• |
the costs involved in filing patent applications and maintaining and enforcing patents or defending against claims or infringements raised by third parties; |
|
|
• |
the time and costs involved in obtaining regulatory approval for our ETB candidates and any delays we may encounter as a result of evolving regulatory requirements or adverse results with respect to any of these ETB candidates; |
|
|
• |
any licensing or milestone fees we might have to pay during future development of our current or any future ETB candidates; |
|
|
• |
selling and marketing activities undertaken in connection with the anticipated commercialization of our current or any future ETB candidates and costs involved in the creation of an effective sales and marketing organization; and |
|
|
• |
the amount of revenues, if any, we may derive either directly or in the form of royalty payments from future sales of our ETB candidates, if approved. |
Identifying potential ETB candidates and conducting pre-clinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. In addition, our ETB candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of drugs or biologics that we do not expect to be commercially available for many years, if ever. Accordingly, we will need to obtain substantial additional funds to achieve our business objectives.
Adequate additional funds may not be available to us on acceptable terms, or at all. To the extent that we raise additional capital through the sale of equity or convertible debt securities, stockholders’ ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect their rights as stockholders. Additional debt financing and equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends and may require the issuance of warrants, which could potentially dilute stockholders’ ownership interest.
If we raise additional funds through collaborations, governmental grants, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or ETB candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development programs or any future
26
commercialization efforts or grant rights to develop and market ETB candidates that we would otherwise prefer to develop and market ourselves.
Critical Accounting Policies and Use of Estimates
Our discussion and analysis of our financial condition and results of operations are based on our unaudited condensed consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America for interim financial information. The preparation of these unaudited condensed consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses based on historical experience and on various assumptions that we believe to be reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions. For further information on our critical accounting policies, see the discussion of critical accounting policies in our Annual Report on Form 10-K for the year ended December 31, 2022, which we filed with the SEC on March 30, 2023.
Recently Adopted Accounting Pronouncements
For a discussion of recently adopted accounting pronouncements see the discussion in our Annual Report on Form 10-K for the year ended December 31, 2022, which we filed with the SEC on March 30, 2023.
Recent Accounting Pronouncements Not Yet Adopted
For a discussion of recently issued accounting pronouncements and interpretations not yet adopted by us, see Note 1, “Organization and Summary of Significant Accounting Policies”, to our unaudited condensed financial statements for the quarter ended March 31, 2023, included in this Quarterly Report on Form 10-Q.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
ITEM 4. CONTROLS AND PROCEDURES
Evaluation of disclosure controls and procedures.
Our management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures at March 31, 2023. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to its management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures at March 31, 2023, our principal executive officer and principal financial officer concluded that the disclosure controls and procedures were effective as of such date.
Changes in internal controls over financial reporting.
There were no changes in our internal control over financial reporting identified in connection with the evaluation required by Rule 13a-15(d) and 15d-15(d) of the Exchange Act that occurred during the three months ended March 31, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
27
PART II. OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
From time to time, we are subject to various legal proceedings, claims and administrative proceedings that arise in the ordinary course of our business activities. Although the results of the litigation and claims cannot be predicted with certainty, as of the date of this report, we do not believe we are party to any claim, proceeding or litigation the outcome of which, if determined adversely to us, would individually or in the aggregate be reasonably expected to have a material adverse effect on our business. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
ITEM 1A. RISK FACTORS
We have identified the following risks and uncertainties that may have a material adverse effect on our business, financial condition or results of operations. The risks described below are not the only ones we face. Additional risks not presently known to us or other factors not perceived by us to present significant risks to our business at this time may also significantly impair our business operations. Our business could be harmed by any of these risks. Investing in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below, together with all other information contained in this Quarterly Report on Form 10-Q, including our condensed consolidated financial statements and the related notes, before making any decision to purchase our common stock. If any of the possible adverse events described below actually occurs, we may be unable to conduct our business as currently planned and our prospects, financial condition, operating results and cash flows could be materially harmed. In addition, the trading price of our common stock could decline due to the occurrence of any of the events described below, and you may lose all or part of your investment. In assessing these risks, you should refer to the other information contained in this Quarterly Report on Form 10-Q, including our condensed consolidated financial statements and related notes.
.
Summary Risk Factors
We are subject to a number of risks that if realized could affect our business, financial condition, results of operations and cash flows. As a clinical stage biopharmaceutical company, certain elements of risk are inherent to our business. Accordingly, we encounter risks as part of the normal course of our business. Some of the more significant challenges and risks include the following:
|
|
• |
We have identified conditions and events that raise substantial doubt about our ability to continue as a going concern, including our continued compliance with the financial covenant in our debt facility, and our ability to avoid triggering an event of default related to, our solvency under our debt facility beyond the third quarter of 2023. Our independent registered public accounting firm has included an explanatory paragraph relating to our ability to continue as a going concern in its report on our audited consolidated financial statements for the year ended December 31, 2022 included in our Annual Report on Form 10-K filed with the SEC on March 30, 2023. |
|
|
• |
We may be unsuccessful in raising the capital necessary to address our going concern issues, or if we are successful, it may be on terms that are highly dilutive to existing stockholders. |
|
|
• |
If we fail to execute successfully on our recently announced strategic reprioritization and restructuring, our business prospects and our financial condition may be adversely affected. Further, the restructuring could result in disruptions to our business during transitional periods and thereafter. |
|
|
• |
A delisting of our common stock from Nasdaq could adversely affect our ability to raise additional capital through the public or private sale of equity securities and for investors to dispose of, or obtain accurate quotations as to the market value of, our common stock. |
|
|
• |
We have incurred losses since inception, have a limited operating history on which to assess our business, and anticipate that we will continue to incur significant losses for the foreseeable future. |
|
|
• |
We have never generated any revenue from product sales and may never become profitable. |
|
|
• |
Manufacturing difficulties, disruptions or delays could limit supply of our drug or biologic candidates and adversely affect our clinical trials. |
|
|
• |
Clinical trials are costly, time consuming and inherently risky, and we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities, and may never obtain regulatory approval for, or successfully commercialize certain or any of our drug or biologic candidates. |
28
|
|
• |
The approach we are taking to discover and develop next generation immunotoxin therapies, also commonly known as engineered toxin bodies (“ETBs”) is unproven and may never lead to marketable products. |
|
|
• |
We are heavily dependent on the success of our drug or biologic candidates, the most advanced of which is in the early stages of clinical development. |
|
|
• |
Our drug or biologic candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial viability of an approved label, or result in significant negative consequences following marketing approval, if any. |
|
|
• |
Product development involves a lengthy and expensive process with an uncertain outcome, and results of earlier preclinical studies and clinical trials may not be predictive of future clinical trial results. |
|
|
• |
We may face potential product liability, and, if successful claims are brought against us, we may incur substantial liability and costs. |
|
|
• |
Biologics carry unique risks and uncertainties, which could have a negative impact on future results of operations. |
|
|
• |
Even if we obtain regulatory approval for a product, we will remain subject to ongoing regulatory requirements. Maintaining compliance with ongoing regulatory requirements may result in significant additional expense to us, and any failure to maintain such compliance could subject us to penalties and cause our business to suffer. |
|
|
• |
Healthcare legislative reform measures may have a material adverse effect on our business, financial condition or results of operations. |
|
|
• |
Our ability to compete effectively may decline if we are unable to establish intellectual property rights or if our intellectual property rights are inadequate to protect our ETB technology, present and future drug or biologic candidates and related processes for our developmental pipeline. |
|
|
• |
We rely on third parties to conduct our clinical trials, manufacture our drug or biologic candidates and perform other services and if such third parties do not successfully carry out their contractual duties, meet expected timelines, or otherwise conduct the trials as required or perform and comply with regulatory requirements, we may not be able to successfully complete clinical development, obtain regulatory approval or commercialize our drug or biologic candidates when expected or at all, and our business could be substantially harmed. |
|
|
• |
We have entered into the BMS Collaboration Agreement with Bristol Myers Squibb and, pursuant to the terms of that agreement, could become dependent on Bristol Myers Squibb for development, manufacturing, regulatory and commercialization activities with respect to certain of our ETB products directed to multiple targets. |
|
|
• |
We face substantial competition, and our competitors may discover, develop or commercialize drugs faster or more successfully than we do. |
|
|
• |
We may not be successful in any efforts to identify, license, discover, develop or commercialize additional drug or biologic candidates. |
|
|
• |
We rely significantly on information technology and any failure, inadequacy, interruption or security lapse of that technology or loss of data, including any cyber security incidents, could compromise sensitive information related to our business, prevent us from accessing critical information or expose us to liability which could harm our ability to operate our business effectively and adversely affect our business and reputation. |
The above list is not exhaustive, and we face additional challenges and risks. Please carefully consider all of the information in this Form 10-Q including matters set forth in this “Risk Factors” section.
29
Risks Related to Our Financial Condition and Capital Requirements
We have identified conditions and events that raise substantial doubt about our ability to continue as a going concern, including our continued compliance with the financial covenant under our debt facility, and the ability to avoid triggering an event of default related to, our solvency under our debt facility beyond the third quarter of 2023. Our independent registered public accounting firm has included an explanatory paragraph relating to our ability to continue as a going concern in its report on our audited consolidated financial statements for the year ended December 31, 2022 included in our Annual Report on Form 10-K filed with the SEC on March 30, 2023.
We believe there is substantial doubt about our ability to continue as a going concern as of the date of this Quarterly Report on Form 10-Q. See Note 1 to our financial statements appearing elsewhere in this Quarterly Report on Form 10-Q for additional information on our assessment. This substantial doubt relates to our future compliance with the financial covenant in our Loan and Security Agreement with K2 HealthVentures LLC (the “K2 Loan and Security Agreement”) which requires us to certify monthly that we have cash, cash equivalents and marketable securities of at least five times our cash monthly burn as defined in the agreement (the “Financial Covenant”), as well as our ability to avoid triggering an event of default related to our solvency (an “Insolvency Event of Default”) under the K2 Loan and Security Agreement. Currently, based on anticipated cost-savings from the Restructuring, we anticipate continued compliance with the Financial Covenant and continued ability to avoid triggering an Insolvency Event of Default late into the third quarter of 2023. However, we will require additional funding in order to meet our covenant requirements and ongoing operations.
If we cannot raise additional capital by then to maintain ourselves in compliance or negotiate an amendment to the Financial Covenant or the Insolvency Events of Default, then we will be in default of the K2 Loan and Security Agreement and the repayment of our indebtedness may be accelerated in full by K2 HealthVentures LLC. At March 31, 2023, we had cash, cash equivalents, and marketable securities of $41.7 million, including borrowings of $35.0 million under the K2 Loan and Security Agreement whose scheduled maturity date for repayment is June 1, 2024, but a default of the Financial Covenant or an Insolvency Event of Default may trigger accelerated repayment.
There can no assurances that we will be able to raise sufficient capital to fund ongoing operations and maintain the Financial Covenant and avoid triggering an Insolvency Event of Default beyond the third quarter of 2023 and/or be successful at negotiating an amendment to the K2 Loan and Security Agreement. If we are unable to obtain additional capital and continue as a going concern, we might have to liquidate our assets and the values we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our financial statements.
Our lack of cash resources and our conclusion that we may be unable to continue as a going concern may materially adversely affect our share price and our ability to raise new capital or to enter into critical contractual relations with third parties.
We may be unsuccessful in raising the capital necessary to address our going concern issues, or if we are successful, it may be on terms that are highly dilutive to existing stockholders.
Historically, we funded our operations by raising capital from external sources, especially through the sale of common stock and our borrowings under the K2 Loan and Security Agreement. However, we are currently facing significant challenges to our ability to raise capital through the sale of common stock, including the following factors:
|
|
• |
in general, it is difficult for development stage companies to raise capital under current market conditions, especially those with early stage programs like ours; |
|
|
• |
the perception that we may be unable to continue as a going concern may impede our ability to attract further equity investment; |
|
|
• |
our common stock has been trading below $1.00 per share since July 2022 (on May 8, 2023, the closing price was $0.4500 per share) and, although we received an extension until August 28, 2023 to regain compliance on the bid price and stockholders’ equity requirements for the Nasdaq Capital Market, we may still be subject to the potential delisting of our common stock if we do not return to compliance by this date. The potential delisting of our common stock from Nasdaq could adversely affect our ability to raise additional capital through the public or private sale of equity securities; and |
|
|
• |
we are currently subject to the “baby shelf” limitations on our potential use of our shelf registration statement, which limits such use to an offering size of no more than $5.7 million. However, this limitation would not affect our ability to raise capital in ways other than our shelf registration statement, such as private placements and PIPEs, if investor demand exists for such offerings by us. |
30
Given these factors, there can be no assurances we will be successful at raising sufficient capital to address our going concern issues. However, if we are successful, it may be on terms that are very highly dilutive to existing stockholders.
If we fail to execute successfully on our recently announced strategic reprioritization and restructuring, our business prospects and our financial condition may be adversely affected. Further, the Restructuring could result in disruptions to our business during transitional periods and thereafter.
There can be no assurances that we will be successful at executing on this strategic reprioritization or that the Restructuring will achieve the cost savings, operating efficiencies or other benefits that we may initially expect, which underlie our current cash runway expectations and our projection that we will remain in compliance with the Financial Covenant under the K2 Loan and Security Agreement and avoid triggering an Insolvency Event of Default under the K2 Loan and Security Agreement late into the third quarter of 2023. The restructuring activities may also result in a loss of continuity, accumulated knowledge and inefficiency during transitional periods and thereafter. In addition, internal restructurings can require a significant amount of time and focus from management and other employees, which may divert attention from operations. Further, the Restructuring may result in unexpected expenses or liabilities and/or write-offs. If the Restructuring fails to achieve some or all of the expected cost-savings and benefits therefrom, our cash resources may not last as long as estimated and our business, results of operations and financial condition could be materially and adversely affected.
A delisting of our common stock from Nasdaq could adversely affect our ability to raise additional capital through the public or private sale of equity securities and for investors to dispose of, or obtain accurate quotations as to the market value of, our common stock.
If our common stock is ultimately delisted by Nasdaq, our common stock may be eligible to trade on the OTC Bulletin Board or another over-the-counter market. Any such alternative would likely result in it being more difficult for us to raise additional capital through the public or private sale of equity securities and for investors to dispose of, or obtain accurate quotations as to the market value of, our common stock. In addition, there can be no assurance that our common stock would be eligible for trading on any such alternative exchange or markets.
Unless our common stock is listed on a national securities exchange, such as the Nasdaq Capital Market, our common stock may also be subject to the regulations regarding trading in “penny stocks,” which are those securities trading for less than $5.00 per share, and that are not otherwise exempted from the definition of a penny stock under other exemptions provided for in the applicable regulations. The following is a list of the general restrictions on the sale of penny stocks:
|
|
• |
Before the sale of penny stock by a broker-dealer to a new purchaser, the broker-dealer must determine whether the purchaser is suitable to invest in penny stocks. To make that determination, a broker-dealer must obtain, from a prospective investor, information regarding the purchaser’s financial condition, investment experience, and objectives. Subsequently, the broker-dealer must deliver to the purchaser a written statement setting forth the basis of the suitability finding and obtain the purchaser’s signature on such statement. |
|
|
• |
A broker-dealer must obtain from the purchaser an agreement to purchase the securities. This agreement must be obtained for every purchase until the purchaser becomes an “established customer.” |
|
|
• |
The Securities Exchange Act of 1934, as amended (the “Exchange Act”) requires that before effecting any transaction in any penny stock, a broker-dealer must provide the purchaser with a “risk disclosure document” that contains, among other things, a description of the penny stock market and how it functions, and the risks associated with such investment. These disclosure rules are applicable to both purchases and sales by investors. |
|
|
• |
A dealer that sells penny stock must send to the purchaser, within 10 days after the end of each calendar month, a written account statement including prescribed information relating to the security. |
These requirements can severely limit the liquidity of securities in the secondary market because fewer brokers or dealers are likely to be willing to undertake these compliance activities. If our common stock is not listed on a national securities exchange, the rules and restrictions regarding penny stock transactions may limit an investor’s ability to sell to a third party and our trading activity in the secondary market may be reduced.
We may seek to effect a reverse stock split, subject to obtaining stockholder approval, in order to address the $1.00 minimum bid price requirement under Nasdaq rules. In the event a reverse stock split is implemented, we cannot predict the effect that such reverse stock split would have on the market price for shares of our common stock, and the history of similar reverse stock splits for companies in like circumstances has varied. Some investors may have a negative view of a reverse stock split. Even if such reverse stock split were to have a positive effect on the market price for shares of our common stock, performance of our business and
31
financial results, general economic conditions and the market perception of our business, and other adverse factors which may not be in our control could lead to a decrease in the price of our common stock following such reverse stock split.
We have incurred losses since inception, have a limited operating history on which to assess our business, and anticipate that we will continue to incur significant losses for the foreseeable future.
We are a clinical development-stage biopharmaceutical company with a limited operating history. We currently generate no revenue from sales of any products, and we may never be able to develop or commercialize a drug or biologic candidate. We have incurred net losses in each year since 2009, excluding the current period. The net income attributable to common shareholders was $10.8 million for the three months ended March 31, 2023. At March 31, 2023, we had an accumulated deficit of $433.9 million.
We have devoted substantially all of our financial resources to identify, acquire, and develop our drug or biologic candidates, including conducting clinical trials and providing general and administrative support for our operations. To date, we have financed our operations primarily through the sale of equity securities, debt financing and collaborations. The amount of our future net losses will depend, in part, on the rate of our future expenditures and our ability to obtain funding through equity or debt financings, strategic collaborations or grants. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk.
Failure to maintain effective internal controls in accordance with Section 404 of the Sarbanes-Oxley Act of 2002, as amended (the “Sarbanes-Oxley Act”) could have a material adverse effect on our stock price.
Section 404 of the Sarbanes-Oxley Act and the related rules and regulations of the SEC require annual management assessments of the effectiveness of our internal control over financial reporting. If we fail to maintain the adequacy of our internal control over financial reporting, as such standards are modified, supplemented or amended from time to time, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act and the related rules and regulations of the SEC. If we cannot favorably assess the effectiveness of our internal control over financial reporting, investor confidence in the reliability of our financial reports may be adversely affected, which could have a material adverse effect on our stock price.
We have never generated any revenue from product sales and may never become profitable.
We have no products approved for commercialization and have never generated any revenue. Our ability to generate revenue and achieve profitability depends on our ability, alone or with strategic collaboration partners, to successfully complete the development of, and obtain the regulatory and marketing approvals necessary to commercialize one or more of our drug or biologic candidates. We do not anticipate generating revenue from product sales for the foreseeable future. Our ability to generate future revenue from product sales depends heavily on our success in many areas, including but not limited to:
|
|
• |
completing research and development of one or more of our drug or biologic candidates; |
|
|
• |
obtaining regulatory and marketing approvals for one or more of our drug or biologic candidates; |
|
|
• |
manufacturing one or more drug or biologic candidates and establishing and maintaining supply and manufacturing relationships with third parties that are commercially feasible; |
|
|
• |
marketing, launching and commercializing one or more drug or biologic candidates for which we obtain regulatory and marketing approval, either directly or with a collaborator or distributor; |
|
|
• |
gaining market acceptance of one or more of our drug or biologic candidates as treatment options; |
|
|
• |
meeting our supply needs in sufficient quantities to meet market demand for our drug or biologic candidates, if approved; |
|
|
• |
addressing any competing products; |
|
|
• |
protecting, maintaining and enforcing our intellectual property rights, including patents, trade secrets and know-how; |
|
|
• |
negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter; |
|
|
• |
obtaining reimbursement or pricing for one or more of our drug or biologic candidates that supports profitability; |
|
|
• |
retaining qualified personnel. |
Even if one or more of the drug or biologic candidates that we develop is approved for commercial sale, we anticipate incurring significant costs associated with launching and commercializing any approved drug or biologic candidate. We also will have to further develop or acquire manufacturing capabilities or continue to contract with contract manufacturing organizations (“CMOs”), in order to
32
continue development and potential commercialization of our drug or biologic candidates. For instance, if our costs of manufacturing our drug products are not commercially feasible, then we will need to develop or procure our drug products in a commercially feasible manner to successfully commercialize any future approved product, if any. Additionally, if we are not able to generate revenue from the sale of any approved products, we may never become profitable.
We hold a portion of our cash and cash equivalents that we use to meet our working capital and operating expense needs in deposit accounts that could be adversely affected if the financial institutions holding such funds fail.
We hold a portion of cash and cash equivalents that we use to meet our working capital and operating expense needs in deposit accounts. The balance held in these accounts may exceed the Federal Deposit Insurance Corporation (“FDIC”) standard deposit insurance limit of $250,000. If a financial institution in which we hold such funds fails or is subject to significant adverse conditions in the financial or credit markets, we could be subject to a risk of loss of all or a portion of such uninsured funds or be subject to a delay in accessing all or a portion of such uninsured funds. Any loss or lack of access to these funds could adversely impact our short-term liquidity and ability to meet our operating expense obligations.
Changes in interpretation or application of U.S. generally accepted accounting principles (“U.S. GAAP”) may adversely affect our operating results.
We prepare our consolidated financial statements to conform to U.S. GAAP. These principles are subject to interpretation by the Financial Accounting Standards Board (“FASB”) American Institute of Certified Public Accountants, the SEC and various other regulatory and accounting bodies. A change in interpretations of, or our application of, these principles can have a significant effect on our reported results and may even affect our reporting of transactions completed before a change is announced. In addition, when we are required to adopt new accounting standards, our methods of accounting for certain items may change, which could cause our results of operations to fluctuate from period to period and make it more difficult to compare our financial results to prior periods.
Risks Related to the Development of Our Drug or Biologic Candidates
Manufacturing difficulties, disruptions or delays could limit supply of our drug or biologic candidates and adversely affect our clinical trials.
We currently have a current good manufacturing practices (“cGMP”) manufacturing facility and we have developed the capability to manufacture drug or biologic candidates for use in the conduct of our clinical trials. We may not be able to manufacture drug or biologic candidates or there may be substantial technical or logistical challenges to supporting manufacturing demand for drug or biologic candidates. We may also fail to comply with cGMP requirements and standards which would require us to not utilize the manufacturing facility to make clinical trial supply.
We plan to rely in part on third-party contract manufacturers, and their responsibilities will include purchasing from third-party suppliers the materials necessary to produce our drug or biologic candidates for our clinical trials and to support future regulatory approval. We expect there to be a limited number of suppliers for some of the raw materials that we expect to use to manufacture our drug or biologic candidates, and we may not be able to identify alternative suppliers to prevent a possible disruption of the manufacture of our drug or biologic candidates for our clinical trials, and, if approved, ultimately for commercial sale.
Although we generally do not expect to begin a clinical trial unless we believe we have a sufficient supply of a drug or biologic candidate to complete the trial, any significant delay or discontinuity in the supply of a drug or biologic candidate, or the raw materials or other material components used in the manufacture of the drug or biologic candidate, could delay completion of our clinical trials and potential timing for regulatory approval of our drug or biologic candidates, which would materially harm our business and results of operations. We do not yet have sufficient information to reliably estimate the cost of the commercial manufacturing of our drug or biologic candidates and our current costs to manufacture our drug products may not be commercially feasible, and the actual cost to manufacture our drug or biologic candidates could materially and adversely affect the commercial viability of our drug or biologic candidates. As a result, we may never be able to develop a commercially viable product.
In addition, as a drug or biologic candidate manufacturer with one manufacturing facility, we are exposed to the following additional risks:
|
|
• |
limited capacity of manufacturing facilities; |
|
|
• |
contamination of drug or biologic candidates in the manufacturing process; |
|
|
• |
events that affect, or have the potential to affect, general economic conditions, including but not limited to political unrest, global trade wars, inflation, natural disasters, acts of war, terrorism, or disease outbreaks, such as the conflict in Ukraine and the COVID-19 pandemic; |
33
|
|
|
|
• |
labor disputes or shortages, including the effects of health emergencies, epidemics, pandemics, such as the COVID-19 pandemic, or natural disasters; |
|
|
• |
failure to ensure compliance with regulatory requirements; |
|
|
• |
changes in forecasts of future demand; |
|
|
• |
timing and actual number of production runs and production success rates and yields; |
|
|
• |
contractual disputes with our suppliers and contract manufacturers; |
|
|
• |
timing and outcome of product quality testing; |
|
|
• |
power failures and/or other utility failures; |
|
|
• |
disruptions or restrictions on the ability of our, our collaborators’, or our suppliers’ personnel to travel that could result in temporary closures of our facilities or the facilities of our collaborators or suppliers; |
|
|
• |
breakdown, failure, substandard performance or improper installation or operation of equipment; |
|
|
• |
following New Drug Application (“NDA”) or Biologics License Application (“BLA”) approval, a change in the manufacturing site would require additional approval from the FDA, which could require new testing and compliance inspections, and we carry the risk of non-compliance with such inspections; |
|
|
• |
we may be unable to timely formulate and manufacture our product or produce the quantity and quality required to meet our clinical and commercial needs, if any; and |
|
|
• |
as a drug or biologic candidate manufacturer, we are subject to ongoing periodic unannounced inspection by the FDA and some state agencies to ensure strict compliance with cGMPs and other U.S. and corresponding foreign requirements, and we carry the risk of non-compliance with these regulations and standards. |
Each of these risks could delay our clinical trials, the marketing approval, if any, of our drug or biologic candidates or the commercialization of our drug or biologic candidates or result in higher costs or deprive us of potential product revenue. In addition, we rely on third parties to perform release testing on our drug or biologic candidates prior to delivery to clinical sites participating in our clinical trials. If these tests are not appropriately conducted and test data are not reliable, subjects participating in our clinical trials, or patients treated with our products, if any are approved in the future, could be put at risk of serious harm, which could result in product liability suits.
Clinical trials are costly, time consuming and inherently risky, and we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities, and may never obtain regulatory approval for, or successfully commercialize certain or any of our drug or biologic candidates.
Clinical development is expensive, time consuming and involves significant risk. We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical trials can occur at any stage of development. Events that may prevent successful or timely completion of clinical development include but are not limited to:
|
|
• |
potential delays in patient enrollment for our clinical trials due to public health emergencies or pandemics, natural disasters, staffing shortages, or other events, which may affect our ability to initiate and/or complete preclinical studies, conduct ongoing clinical trials, and delay initiation of planned and future clinical trials; |
|
|
• |
inability to generate satisfactory preclinical, toxicology or other in vivo or in vitro data or to develop diagnostics capable of supporting the initiation or continuation of clinical trials; |
|
|
• |
delays in reaching agreement on acceptable terms with clinical research organizations (“CROs”), and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical trial sites; |
|
|
• |
delays or failure in obtaining required an institutional review board (“IRB”) approval at each clinical trial site; |
|
|
• |
failure to obtain or delays in obtaining a permit from regulatory authorities to conduct a clinical trial; |
|
|
• |
delays in recruiting or failure to recruit sufficient eligible volunteers or subjects in our clinical trials; |
|
|
• |
failure by clinical trial sites or CROs or other third parties to adhere to clinical trial requirements; |
34
|
|
• |
failure by our clinical trial sites, CROs or other third parties to perform in accordance with the good clinical practices requirements of the FDA or applicable foreign regulatory guidelines; |
|
|
• |
subjects withdrawing from our clinical trials; |
|
|
• |
adverse events or other issues of concern significant enough for the FDA, or comparable foreign regulatory authority, to put a clinical trial or an IND on clinical hold; |
|
|
• |
occurrence of adverse events associated with our drug or biologic candidates; |
|
|
• |
changes in regulatory requirements and guidance that require amending or submitting new clinical protocols; |
|
|
• |
the cost of clinical trials of our drug or biologic candidates; |
|
|
• |
negative or inconclusive results from our clinical trials which may result in us deciding, or regulators requiring us, to conduct additional clinical trials or abandon development programs in other ongoing or planned indications for a drug or biologic candidate; and |
|
|
• |
delays in reaching agreement on acceptable terms with third-party manufacturers or an inability to manufacture sufficient quantities of our drug or biologic candidates for use in clinical trials. |
Any inability to successfully complete clinical development and obtain regulatory approval for one or more of our drug or biologic candidates could result in additional costs to us or impair our ability to generate revenue. In addition, if we make manufacturing or formulation changes to our drug or biologic candidates, we may need to conduct additional nonclinical studies and/or clinical trials to show that the results obtained from such new formulation are consistent with previous results. Clinical trial delays, including those caused by the COVID-19 pandemic, could also shorten any periods during which our drug or biologic candidates have patent protection and may allow competitors to develop and bring products to market before we do, which could impair our ability to successfully commercialize our drug or biologic candidates and may harm our business and results of operations.
Additionally, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of marketing approval for our drug or biologic candidates which would materially harm our business. The FDA may disagree with our clinical trial design and our interpretation of data from clinical trials, or may change the requirements for approval even after it has reviewed and commented on the design for our clinical trials. For example, the FDA published guidance in January 2023 on “Project Optimus,” an initiative to reform dose selection in oncology drug development with the goal of optimizing the design of early dose-finding trials. If the FDA does not believe we have sufficiently demonstrated that the selected doses for our drug or biologic candidates maximize not only the efficacy of such candidate, but the safety and tolerability as well, our ability to initiate new studies may be delayed and our costs may be increased. Even if we conducted the additional studies or generated the additional information requested, the FDA could disagree that we have satisfied the agency’s requirements, all of which would cause significant delays and expense to our programs.
The approach we are taking to discover and develop next generation immunotoxin therapies, also commonly known as ETBs, is unproven and may never lead to marketable products.
The scientific discoveries that form the basis for our efforts to discover and develop our drug or biologic candidates are relatively recent. To date, neither we nor any other company has received regulatory approval to market products utilizing ETBs. The scientific evidence to support the feasibility of developing drugs based on these discoveries is both preliminary and limited. Successful development of ETB therapeutic products by us will require addressing a number of issues, including identifying appropriate receptor targets, screening for and selecting potent and safe ETB drug or biologic candidates, developing a commercially feasible manufacturing process, successfully completing all required preclinical studies and clinical trials, successfully implementing all other requirements that may be mandated by regulatory agencies from clinical development through post-marketing periods, ensuring intellectual property protection in any territory where an ETB product may be commercialized and commercializing an ETB product successfully in a competitive product landscape. In addition, any drug or biologic candidates that we develop may not demonstrate in patients the biological or pharmacological properties ascribed to them in laboratory and preclinical testing, and they may interact with human biological systems in unforeseen, ineffective or even harmful ways. If we do not successfully develop and commercialize one or more drug or biologic candidates based upon this scientific approach, we may not become profitable and the value of our common stock may decline.
35
Further, our focus on ETB technology for developing drug or biologic candidates as opposed to multiple, more proven technologies for drug development increases the risk associated with our business. If we are not successful in developing an approved product using ETB technology, we may not be able to identify and successfully implement an alternative product development strategy. In addition, work by other companies pursuing similar immunotoxin technologies may encounter setbacks and difficulties that regulators and investors may attribute to our drug or biologic candidates, whether appropriate or not.
Our clinical trial of MT-0169 is currently subject to a partial clinical hold imposed by the FDA. A clinical hold on any of our clinical trials could result in delays of our clinical development timeline.
On April 7, 2023, we announced that the FDA notified us that MT-0169 clinical study had been placed on partial clinical hold based on previously disclosed cardiac adverse events noted in two patients dosed at 50 mcg/kg that prompted a dose reduction to 5 mcg/kg in early 2022. Under the partial clinical hold, patients already enrolled in the MT-0169 clinical study can continue treatment, but no new patients may be enrolled until the partial hold is lifted by the FDA. There can be no assurance that the FDA will lift the partial clinical hold; furthermore, the FDA may expand the scope of the partial clinical hold in the future or impose a full hold. If the FDA does not lift the partial clinical hold in the near future or at all, our clinical development of MT-0169 will be materially and adversely delayed and impaired. There can be no assurance that our current or future clinical trials will not be subject to additional partial or full clinical holds, which could delay or impair the commencement and completion of our clinical trials and the regulatory approval of our drug or biologic candidates.
We are working towards addressing the partial clinical hold by providing the information requested by the FDA, including, but not limited to, narratives on the two patients who experienced cardiotoxicity at 50 mcg/kg, justification for the revised dose of 5 mcg/kg, and data evaluating the clinical benefit-to-risk ratio seen with the lower doses of MT-0169. Following submission of such requests to the FDA, we will seek agreement from FDA to remove the partial clinical hold. We submitted our responses to the partial clinical hold to the FDA in May 2023. There can be no assurance with respect to our ability to remove the partial clinical hold, or the timing thereof.
We are heavily dependent on the success of our drug or biologic candidates, the most advanced of which is in the early stages of clinical development. Our ETB therapeutic drug or biologic candidates are based on a relatively novel technology, which makes it difficult to predict the time and cost of development and of subsequently obtaining regulatory approval, if at all. Some of our drug or biologic candidates have produced results in preclinical settings to date and we cannot give any assurance that we will generate additional nonclinical and clinical data for any of our drug or biologic candidates that are sufficient to receive regulatory approval in our planned indications, which will be required before they can be commercialized. To date, no ETB products have been approved for marketing in the United States or elsewhere.
We have concentrated our research and development efforts to date on a limited number of drug or biologic candidates based on our ETB therapeutic platform and identifying our initial targeted disease indications. We have invested substantially all of our efforts and financial resources to identify, acquire and develop our portfolio of drug or biologic candidates. Our future success is dependent on our ability to successfully further develop, obtain regulatory approval for, and commercialize one or more drug or biologic candidates. We currently generate no revenue from sales of any products, and we may never be able to develop or commercialize a drug or biologic candidate. Our ETB candidate, MT-6402, is currently being tested in a Phase I study in relapsed/refractory patients with PD-L1 expressing tumors, which began dosing patients in the third quarter of 2021. Our CD38-targeted ETB, MT-0169, has been administered to patients with relapsed/refractory multiple myeloma and non-Hodgkin’s lymphoma in a Phase I trial but was placed on a partial clinical hold by the FDA in April 2023 based upon previously disclosed cardiac adverse events noted in two patients dosed at 50 mcg/kg that prompted a dose reduction to 5 mcg/kg in early 2022. No new patients will be enrolled in this study until all requests for information from the FDA are completed and the partial clinical hold is lifted. Patients already enrolled in the MT-0169 clinical study can continue treatment. The Phase I study for MT-0169 began dosing patients in the first quarter of 2020 was paused in March 2020 due to COVID-19 and was re-initiated during the fourth quarter of 2020. The revised protocol for the Phase I study for MT-0169 began dosing patients in July 2022 and had cleared the 5 mcg/kg and 10 mcg/kg doses. Our ETB candidate MT-8421 is planned to be tested in Phase I study mid-year 2023. There can be no assurance that we will not experience problems or delays in developing our drug or biologic candidates and that such problems or delays will not cause unanticipated costs, or that any such development problems can be solved. Additionally, not all of our clinical and preclinical data to date have been validated and we have no way of knowing if after validation our clinical trial data will be complete and consistent. There can be no assurance that the data that we develop for our drug or biologic candidates in our planned indications will be sufficient to obtain regulatory approval.
36
None of our ETB drug or biologic candidates have advanced into a pivotal clinical trial for our proposed indications and it may be years before any such clinical trial is initiated and completed, if at all. We are not permitted to market or promote any of our drug or biologic candidates before we receive regulatory approval from the FDA or a comparable foreign regulatory authority, and we may never receive such regulatory approval for any of our drug or biologic candidates. We cannot be certain that any of our drug or biologic candidates will be successful in clinical trials or receive regulatory approval. Further, our drug or biologic candidates may not receive regulatory approval even if they are successful in clinical trials. If we do not receive regulatory approvals for our drug or biologic candidates, we may not be able to continue our operations.
Additionally, the FDA and comparable foreign regulatory authorities have relatively limited experience with ETB products. No regulatory authority has granted approval to any person or entity, including us, to market or commercialize ETB product candidates, which may increase the complexity, uncertainty and length of the regulatory approval process for our drug or biologic candidates. If our ETB product candidates fail to prove to be safe, effective or commercially viable, our drug or biologic candidate pipeline would have little, if any, value, which would have a material adverse effect on our business, financial condition or results of operations.
The clinical trial and manufacturing requirements of the FDA, the EMA, and other regulatory authorities, and the criteria these regulators use to determine the safety and efficacy of a drug or biologic candidate, vary substantially according to the type, complexity, novelty and intended use and market of the drug or biologic candidate. The regulatory approval process for novel drug or biologic candidates such as ETB product candidates could be more expensive and take longer than for others, better known or more extensively studied drug or biologic candidates. It is difficult to determine how long it will take or how much it will cost to obtain regulatory approvals for our drug or biologic candidates in either the United States or the European Union or elsewhere or how long it will take to commercialize our drug or biologic candidates, even if approved for marketing. Approvals by the EMA and other regulatory authorities may not be indicative of what the FDA may require for approval, and vice versa, and different or additional preclinical studies and clinical trials may be required to support regulatory approval in each respective jurisdiction. Delay or failure to obtain, or unexpected costs in obtaining, the regulatory approval necessary to bring a drug or biologic candidate to market could decrease our ability to generate sufficient product revenue, and our business, financial condition, results of operations and prospects may be harmed.
The ongoing effects of the COVID-19 pandemic, moreover, including the responses of the federal, international, state and regional governments to the pandemic, could continue to have an impact on the timeline for review and approval of new marketing applications. The FDA announced in July 2022 that remote regulatory assessments of facilities and other alternative approaches developed during the first two years of the pandemic will continue to be used by the agency in order to supplement its in-person inspection program. Congress endorsed the FDA’s approach to remote facility assessments via amendments made to the FDCA as part of the Consolidated Appropriations Act for 2023.
We may have difficulty enrolling, or fail to enroll patients, in our clinical trials given the limited number of patients who have the diseases for which our drug or biologic candidates are being studied, which could delay or prevent clinical trials of our drug or biologic candidates.
Identifying and enrolling patients to participate in clinical trials of our ETB drug or biologic candidates is essential to our success. The timing of our clinical trials depends in part on the rate at which we can recruit patients to participate in clinical trials of our drug or biologic candidates, and we may experience delays in our clinical trials if we encounter difficulties in enrollment, particularly due to public health emergencies or pandemics, natural disasters, staffing shortages, or otherwise.
The eligibility criteria of our ongoing and planned clinical trials may further limit the available eligible trial participants as we require that patients have specific characteristics that we can measure or meet the criteria to assure their conditions are appropriate for inclusion in our clinical trials. We may not be able to identify, recruit and enroll a sufficient number of patients to complete our clinical trials in a timely manner because of the perceived risks and benefits of the drug or biologic candidate under study, the availability and efficacy of competing therapies and clinical trials, and the willingness of physicians to participate in our planned clinical trials. If patients are unwilling to participate in our clinical trials for any reason, the timeline for conducting trials and obtaining regulatory approval of our drug or biologic candidates may be delayed.
If we experience delays in the completion of, or termination of, any clinical trials of our drug or biologic candidates, the commercial prospects of our drug or biologic candidates could be harmed, and our ability to generate product revenue from any of these drug or biologic candidates could be delayed or prevented. In addition, any delays in completing our clinical trials would likely increase our overall costs, impair drug or biologic candidate development and jeopardize our ability to obtain regulatory approval relative to our current plans. Any of these occurrences may harm our business, financial condition, and prospects significantly.
37
Our drug or biologic candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial viability of an approved label, or result in significant negative consequences following marketing approval, if any.
Undesirable side effects caused by our drug or biologic candidates could cause us or regulatory authorities to interrupt, delay, or terminate clinical trials or result in a restrictive label or delay regulatory approval. In addition, our ETB product candidates have been studied in only a limited number of subjects. Based on observations with a similar class of immunotoxins or ETBs, the adverse events (“AEs”) considered to be important or potential risks of MT-6402 include, but are not limited to, cytokine release syndrome (“CRS”), infusion-related reactions (“IRR”), immune-related adverse reactions, hepatotoxicity, acute kidney injury, hematologic toxicity, coagulation and clinical chemistry toxicity, capillary leak syndrome (“CLS”), reproductive risks, and cardiovascular toxicity. The important or potential risks of MT-0169 include, but are not limited to, CRS, skeletal muscle and cardiac injury, CLS, IRR, thrombotic microangiopathy (“TMA”) with glomerular endothelial cell swelling/injury and increased risk of infections.
In addition to the side effects that are known to be associated with MT-6402 and MT-0169, continued clinical trials could reveal higher incidence of side effects or AEs, previously unknown side effects, or side effects having greater severity, which could each or all lead to delays in our clinical programs, including MT-8421, or discontinuation of our trials. Regulatory authorities may suspend or terminate a clinical trial due to a number of factors, including, among other things, failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in cited deficiencies, or the imposition of a clinical hold, study subject safety concerns, adverse effects or events, severe adverse events including death, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions. The occurrence of adverse side effects could jeopardize or preclude our ability to develop, obtain or maintain marketing approval for, or successfully commercialize, market and sell any or all of our product candidates for one or more indications. There is no guarantee that additional or more severe side effects will not be identified through ongoing clinical trials of our drug or biologic candidates for current and other indications. There can be no assurance that other patients treated with MT-6402, MT-8421, MT-0169, or any other of our drug or biologic candidates, will not experience CLS or other serious side effects and there can be no assurance that the FDA, EMA or comparable regulatory authorities in other jurisdictions will not place additional clinical holds on our current or future clinical trials, the result of which could delay or prevent us from obtaining regulatory approval for any or all ETB product candidates. For additional information, see the risk factor titled “Our clinical trial of MT-0169 is currently subject to a partial clinical hold imposed by the FDA. A clinical hold on any of our clinical trials could result in delays of our clinical development timeline.”
Even if approved in the future, MT-6402, MT-8421, MT-0169, or any other of our drug or biologic candidates, may carry boxed warnings or other warnings and precautions. Undesirable side effects and negative results for any of our drug or biologic candidates may negatively impact the development and potential for approval of our drug or biologic candidates for their proposed indications.
Additionally, even if one or more of our drug or biologic candidates receives marketing approval, if we or others later identify undesirable side effects caused by such products, potentially significant negative consequences could result, including but not limited to:
|
|
• |
regulatory authorities may withdraw approvals of such products; |
|
|
• |
regulatory authorities may require additional warnings or new contraindications on the label; |
|
|
• |
we may be required to create a Risk Evaluation and Mitigation Strategies (“REMS”) plan, which could include a medication guide outlining the risks of such side effects for distribution to patients, a communication plan for healthcare providers, or other elements to assure safe use; |
|
|
• |
we may be required to change the way such drug or biologic candidates are distributed or administered, or change the labeling of the drug or biologic candidates; |
|
|
• |
we may be subject to regulatory investigations and government enforcement actions; |
|
|
• |
the FDA or a comparable foreign regulatory authority may require us to conduct additional clinical trials or costly post-marketing testing and surveillance to monitor the safety and efficacy of the product; |
|
|
• |
we may decide to recall such drug or biologic candidates from the marketplace; and |
|
|
• |
our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of a drug or biologic candidate, even if approved, and could significantly harm our business, results of operations, and prospects.
38
Our ETB therapeutic approach is novel and negative public opinion and increased regulatory scrutiny of ETB-based therapies may damage public perception of the safety of our drug or biologic candidates and adversely affect our ability to conduct our business or obtain regulatory approvals for our drug or biologic candidates.
ETB therapy remains a novel technology, with no ETB therapy product approved to date in the United States or elsewhere worldwide. Public perception may be influenced by claims that ETB therapy is unsafe, and ETB therapy may not gain the acceptance of the public or the medical community. In particular, our success will depend upon physicians who specialize in the treatment of the diseases targeted by our drug or biologic candidates prescribing treatments that involve the use of one or more of our approved drug or biologic candidates in lieu of, or in addition to, existing treatments with which they may be familiar and for which more clinical data may be available. More restrictive government regulations or negative public opinion regarding ETB-based drug or biologic candidates could have an adverse effect on our business, financial condition or results of operations and may delay or impair the development and commercialization of our drug or biologic candidates or demand for any products we may develop. Serious adverse events in ETB clinical trials for our competitors’ products, even if not ultimately attributable to the relevant drug or biologic candidates, and the resulting publicity, could result in increased government regulation, unfavorable public perception, potential regulatory delays in the testing or approval of our drug or biologic candidates, stricter labeling requirements for those drug or biologic candidates that are approved and a decrease in demand for any such drug or biologic candidates.
Product development involves a lengthy and expensive process with an uncertain outcome, and results of earlier preclinical studies and clinical trials may not be predictive of future clinical trial results.
We currently have no products approved for sale and we cannot guarantee that we will ever have marketable products. Clinical testing is expensive and generally takes many years to complete, and the outcome is inherently uncertain. Failure can occur at any time during the clinical development process. Clinical trials may produce negative or inconclusive results, and we or any current or future collaboration partners may decide, or regulators may require us, to conduct additional clinical trials or preclinical studies. We will be required to demonstrate with substantial evidence through well-controlled clinical trials that our drug or biologic candidates are safe and effective for use in a diverse population before we can seek marketing approvals for their commercial sale. The results of preclinical studies and early clinical trials of our drug or biologic candidates may not be predictive of the results of larger, later-stage controlled clinical trials. Drug or biologic candidates that have shown promising results in early-stage clinical trials may still suffer significant setbacks or failure in subsequent clinical trials. Our clinical trials to date have been conducted on a small number of subjects in limited numbers of clinical trial sites for a limited number of indications. We will have to conduct larger, well-controlled trials in our proposed indications to verify the results obtained to date and to support any regulatory submissions for further clinical development. A number of companies in the biopharmaceutical industry have suffered significant setbacks or failure in advanced clinical trials due to lack of efficacy or adverse safety profiles despite promising results in earlier, smaller clinical trials. In particular, no ETB-based product candidates have been approved or commercialized in any jurisdiction, and the outcome of our preclinical studies and early-stage clinical trials may not be predictive of the success of later-stage clinical trials.
From time to time, we may publish or report interim or preliminary data from our clinical trials. Interim or preliminary data from clinical trials that we may conduct may not be indicative of the final results of the trial and are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Interim or preliminary data also remain subject to audit and verification procedures that may result in the final data being materially different from the interim or preliminary data. As a result, interim or preliminary data should be viewed with caution until the final data is available.
In some instances, there can be significant variability in safety and efficacy results between different clinical trials of the same drug or biologic candidate due to numerous factors, including changes in trial protocols, differences in size and type of the patient populations, differences in and adherence to the dosing regimen and other trial protocols and the rate of dropout among clinical trial participants. We therefore do not know whether any clinical trials we may conduct will demonstrate consistent or adequate efficacy and safety sufficient to obtain marketing approval to market our drug or biologic candidates.
We may use our financial and human resources to pursue a particular research program or drug or biologic candidate and fail to capitalize on programs or drug or biologic candidates that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and human resources, we may forego or delay pursuit of opportunities with certain programs or drug or biologic candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or more profitable market opportunities. Our spending on current and future research and development programs and future drug or biologic candidates for specific indications may not yield any commercially viable products. We may also enter into additional strategic collaboration agreements to develop and commercialize some of our programs and potential drug or biologic candidates in indications with potentially large commercial markets. If we do not accurately evaluate the commercial potential or target market for a particular drug or biologic candidate, we may relinquish valuable rights to that drug or biologic candidate through strategic collaborations, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such drug or biologic candidate.
39
We may allocate internal resources to a drug or biologic candidate in a therapeutic area in which it would have been more advantageous to enter into a partnering arrangement, or we may enter into supply agreements with third parties that may be costly for us to maintain.
We may face potential product liability, and, if successful claims are brought against us, we may incur substantial liability and costs. If the use or misuse of our drug or biologic candidates harms subjects or is perceived to harm subjects even when such harm is unrelated to our drug or biologic candidates, we could be subject to costly and damaging product liability claims. If we are unable to obtain adequate insurance or are required to pay for liabilities resulting from a claim excluded from, or beyond the limits of, our insurance coverage, a material liability claim could adversely affect our financial condition.
The use or misuse of our drug or biologic candidates in clinical trials and the sale of any products for which we may obtain marketing approval exposes us to the risk of potential product liability claims. Product liability claims might be brought against us by consumers, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our drug or biologic candidates and approved products, if any. There is a risk that our drug or biologic candidates may induce AEs. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs.
Some of our ETB product candidates have shown in clinical trials to induce adverse events. The adverse events considered to be important or potential risks of MT-6402 include, but are not limited to, CRS, IRR, immune-related adverse reactions, hepatotoxicity, acute kidney injury, hematologic toxicity, coagulation and clinical chemistry toxicity, CLS, reproductive risks, and cardiovascular toxicity. The important or potential risks of MT-0169 include, but are not limited to, CRS, skeletal muscle and cardiac injury, CLS, IRR, TMA with glomerular endothelial cell swelling/injury and increased risk of infections.
There is a risk that our future drug or biologic candidates may induce similar or more severe adverse events. Patients with the diseases targeted by our drug or biologic candidates may already be in severe or advanced stages of disease and have both known and unknown significant preexisting and potentially life-threatening health risks. During the course of treatment, subjects may suffer adverse events, including death, for reasons that may be related to our drug or biologic candidates. Such events could subject us to costly litigation, require us to pay substantial amounts of money to injured subjects, delay, negatively impact or end our opportunity to receive or maintain regulatory approval to market our products, or require us to suspend or abandon our commercialization efforts. Even in a circumstance in which an adverse event is unrelated to our drug or biologic candidates, the investigation into the circumstance may be time-consuming or inconclusive. These investigations may delay our regulatory approval process or impact and limit the type of regulatory approvals our drug or biologic candidates receive or maintain. As a result of these factors, a product liability claim, even if successfully defended, could have a material adverse effect on our business, financial condition or results of operations.
Although we have a claims-made product liability insurance covering our clinical trials in the United States for up to $7.0 million per occurrence up to an aggregate limit of $7.0 million, and coverage for our clinical trials outside of the United States, our insurance may be insufficient to reimburse us for any expenses or losses we may suffer. We also will likely be required to increase our product liability insurance coverage for the advanced clinical trials that we plan to initiate. If we obtain marketing approval for any of our drug or biologic candidates, we will need to expand our insurance coverage to include the sale of commercial products. There is no way to know if we will be able to continue to obtain product liability coverage and obtain expanded coverage if we require it, in sufficient amounts to protect us against losses due to liability, on acceptable terms, or at all. We may not have sufficient resources to pay for any liabilities resulting from a claim excluded from, or beyond the limits of, our insurance coverage. Where we have provided indemnities in favor of third parties under our agreements with them, there is also a risk that these third parties could incur liability and bring a claim under such indemnities. An individual may bring a product liability claim against us alleging that one of our drug or biologic candidates causes, or is claimed to have caused, an injury or is found to be unsuitable for consumer use. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability and breach of warranties. Claims also could be asserted under state consumer protection acts. Any product liability claim brought against us, with or without merit, could result in:
|
|
• |
withdrawal of clinical trial volunteers, investigators, subjects or trial sites; |
|
|
• |
the inability to commercialize, or if commercialized, decreased demand for, our drug or biologic candidates; |
|
|
• |
if commercialized, product recalls, limitations on approved indications, marketing or promotional restrictions or the need for product modification; |
|
|
• |
initiation of investigations by regulators or government enforcement bodies; |
|
|
• |
loss of revenues; |
40
|
|
• |
substantial costs of litigation, including monetary awards to subjects or other claimants; |
|
|
• |
liabilities that substantially exceed our product liability insurance, which we would then be required to pay; |
|
|
• |
an increase in our product liability insurance rates or the inability to maintain insurance coverage in the future on acceptable terms, if at all; |
|
|
• |
the diversion of management’s attention from our business; and |
|
|
• |
damage to our reputation and the reputation of our products and our technology. |
Product liability claims may subject us to the foregoing and other risks, which could have a material adverse effect on our business, financial condition or results of operations.
Biologics carry unique risks and uncertainties, which could have a negative impact on future results of operations.
The successful discovery, development, manufacturing and sale of biologics is a long, expensive and uncertain process. There are unique risks and uncertainties with biologics. For example, access to and supply of necessary biological materials, such as cell lines, may be limited and governmental regulations restrict access to and regulate the transport and use of such materials. In addition, the development, manufacturing and sale of biologics is subject to regulations that are often more complex and extensive than the regulations applicable to other pharmaceutical products. Manufacturing biologics, especially in large quantities, is often complex and may require the use of innovative technologies. Such manufacturing also requires facilities specifically designed and validated for this purpose and sophisticated quality assurance and quality control procedures. Biologics are also frequently costly to manufacture because production inputs are derived from living animal or plant material, and some biologics cannot be made synthetically. Failure to successfully discover, develop, manufacture and sell our biological drug or biologic candidates would adversely impact our business and future results of operations.
Our international activities, including clinical trials previously opened abroad, expose us to various risks, any number of which could harm our business.
We are subject to the risks inherent in engaging in business across national boundaries, due in part to our clinical trials, some of which were previously open abroad and may be opened abroad in the future, any one of which could adversely impact our business. In addition to currency fluctuations, these risks include, among other things: economic downturns, pandemics, changes in or interpretations of local law, varying data protection requirements, governmental policy or regulation; restrictions on the transfer of funds into or out of the country; varying tax systems; and government protectionism. One or more of the foregoing factors could impair our current or future operations and, as a result, harm our overall business.
Fluctuations in foreign currency exchange rates could result in changes in our reported financial results.
We incurred, and may incur again in the future, significant expenses denominated in foreign currencies, specifically in connection with our clinical trial sites, several of which were located in various countries outside of the United States. These clinical trial sites invoiced us in the local currency of the site. If we expand internationally, our exposure to currency risks will increase. We do not manage our foreign currency exposure in a manner that would eliminate the effects of changes in foreign exchange rates. Therefore, changes in exchange rates between these foreign currencies and the U.S. dollar will affect our revenues and expenses and could result in exchange losses in any given reporting period. We incur currency transaction risks whenever we enter into either a purchase or a sale transaction using a currency other than the dollar, our functional currency, particularly in our arrangements for the purchase of supplies or licensing and collaboration agreements with partners outside of the United States. We do not engage in foreign currency hedging arrangements for our accounts payable, and, consequently, foreign currency fluctuations may adversely affect our earnings. We may decide to manage this risk by hedging our foreign currency exposure, principally through derivative contracts. Even if we decide to enter into such hedging transactions, we cannot be sure that such hedges will be effective or that the costs of such hedges will not exceed their benefits. Given the volatility of exchange rates, we can give no assurance that we will be able to effectively manage our currency transaction risks or that any volatility in currency exchange rates will not have an adverse effect on our results of operations.
Our business activities may be subject to the Foreign Corrupt Practices Act and similar anti-bribery and anti-corruption laws of other countries in which we operate.
We have conducted studies in international locations and may in the future initiate studies in countries other than the United States. Our business activities may be subject to the Foreign Corrupt Practices Act (“FCPA”) and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we operate. The FCPA generally prohibits offering, promising, giving or authorizing others to give anything of value, either directly or indirectly, to a non-U.S. government official in order to influence
41
official action or otherwise obtain or retain business. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal accounting controls. Our business is heavily regulated and therefore involves significant interaction with public officials, including officials of non-U.S. governments. Additionally, in many other countries, the health care providers who prescribe pharmaceuticals are employed by their governments, and the purchasers of pharmaceuticals are government entities; therefore, our dealings with these prescribers and purchasers are subject to regulation under the FCPA. Recently the SEC and Department of Justice have increased their FCPA enforcement activities with respect to biotechnology and pharmaceutical companies. There is no certainty that all of our employees, agents or contractors, or those of our affiliates, will comply with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers or our employees, the closing down of our facilities, requirements to obtain export licenses, cessation of business activities in sanctioned countries, implementation of compliance programs and prohibitions on the conduct of our business. Any such violations could include prohibitions on our ability to offer our products, if approved, in one or more countries and could materially damage our reputation, our brand, our international expansion efforts, our ability to attract and retain employees and our business, prospects, operating results and financial condition.
Risks Related to Regulatory Approval of Our Drug or Biologic Candidates and Other Legal Compliance Matters
A potential breakthrough therapy designation by the FDA for our drug or biologic candidates may not lead to a faster development or regulatory review or approval process, and it does not increase the likelihood that our drug or biologic candidates will receive marketing approval.
We may seek a breakthrough therapy designation from the FDA for one or more of our drug or biologic candidates. A breakthrough therapy is defined as a drug or biological product that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug or biological product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For drugs or biological products that have been designated as breakthrough therapies, interaction and communication between the FDA and the sponsor of a clinical trial can help to identify the most efficient path for clinical development while minimizing the number of patients placed in ineffective control regimens. Drugs or biologic products designated as breakthrough therapies by the FDA could also be eligible for accelerated approval.
Designation as a breakthrough therapy is within the discretion of the FDA. Accordingly, even if we believe one of our drug or biologic candidates meets the criteria for designation as a breakthrough therapy, the FDA may disagree and instead determine not to make such designation. In any event, the receipt of a breakthrough therapy designation for a drug or biologic candidate may not result in a faster development process, review or approval, compared to drugs or biologics considered for approval under conventional or other accelerated FDA procedures and does not ensure ultimate approval by the FDA. In addition, even if one or more of our drug or biologic candidates qualify and are designated as a breakthrough therapy, the FDA may later decide that the drugs or biological products no longer meet the conditions for designation and the designation may be rescinded.
MT-6402 has been granted Fast Track designation by the FDA and we may seek Fast Track designation for one or more of our other drug or biologic candidates in the future. Even if we apply for Fast Track designation in the future, we might not receive such designation, and even if we do, such designation may not actually lead to a faster development or regulatory review or approval process, and further, such designation could be withdrawn by the FDA.
If a drug or biologic candidate is intended for the treatment of a serious condition and nonclinical or clinical data demonstrate the potential to address an unmet medical need for this condition, a product sponsor may request an FDA Fast Track designation from the FDA. If we seek Fast Track designation for a drug or biologic candidate, we may not receive it from the FDA. However, even if we receive Fast Track designation, Fast Track designation does not ensure that we will receive marketing approval or that approval will be granted within any particular time frame. We may not experience a faster development or regulatory review or approval process with Fast Track designation compared to conventional FDA procedures. In addition, the FDA may withdraw Fast Track designation if the designation is no longer supported by data from our clinical development program. Fast Track designation alone does not guarantee qualification for the FDA’s priority review procedures. In November 2021, MT-6402 was granted Fast Track designation for the treatment of patients with advanced NSCLC expressing PD-L1.
42
Even if we obtain regulatory approval for a product, we will remain subject to ongoing regulatory requirements. Maintaining compliance with ongoing regulatory requirements may result in significant additional expense to us, and any failure to maintain such compliance could subject us to penalties and cause our business to suffer.
If any of our drug or biologic candidates are approved, we will be subject to ongoing regulatory requirements with respect to manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing clinical trials and submission of safety, efficacy and other post-approval information, including both federal and state requirements in the United States and requirements of comparable foreign regulatory authorities.
Manufacturers and manufacturers’ facilities are required to continuously comply with FDA and comparable foreign regulatory authority requirements, including ensuring that quality control and manufacturing procedures conform to cGMP regulations and corresponding foreign regulatory manufacturing requirements. As such, we and our CMOs will be subject to continual review and inspections to assess compliance with cGMP and adherence to commitments made in any BLA, NDA or other marketing authorization application.
Any regulatory approvals that we receive for our drug or biologic candidates may be subject to limitations on the approved indicated uses for which the drug or biologic candidate may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase IV clinical trials, and surveillance to monitor the safety and efficacy of the drug or biologic candidate. In addition, if the FDA, EMA or a comparable foreign regulatory authority approves any of our drug or biologic candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and record keeping for the products will be subject to extensive and ongoing regulatory requirements. Any new legislation addressing drug safety issues could result in delays in product development or commercialization, or increased costs to assure compliance. If our original marketing approval for a drug or biologic candidate was obtained through an accelerated approval pathway, we could be required to conduct a successful post-marketing clinical trial in order to confirm the clinical benefit for our products. An unsuccessful post-marketing clinical trial or failure to complete such a trial could result in the withdrawal of marketing approval.
We must also comply with requirements concerning advertising and promotion for any of our drug or biologic candidates for which we hope to obtain marketing approval. Promotional communications with respect to prescription drugs and biologics are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved labeling. If we are not able to comply with post-approval regulatory requirements, we could have marketing approval for any of our products withdrawn by regulatory authorities and our ability to market any future products could be limited, which could adversely affect our ability to achieve or sustain profitability. Thus, the cost of compliance with post-approval regulations may have a negative effect on our operating results and financial condition.
In addition, later discovery of previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, or failure to comply with applicable regulatory requirements may result in a variety of risks. For example, a regulatory agency or enforcement authority may, among other things:
|
|
• |
impose restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
|
|
• |
impose requirements to conduct post-marketing studies or clinical trials; |
|
|
• |
issue warning or untitled letters if the regulator is the FDA, or comparable notice of violations from foreign regulatory authorities; |
|
|
• |
issue consent decrees, injunctions or impose civil or criminal penalties; |
|
|
• |
require the payment of fines, restitution or disgorgement of profits or revenues; |
|
|
• |
suspend or withdraw regulatory approval; |
|
|
• |
suspend any of our ongoing clinical trials; |
|
|
• |
refuse to approve pending applications or supplements to approved applications submitted by us; |
|
|
• |
impose restrictions on our operations, including closing our or our CMOs’ manufacturing or analytical testing facilities; or |
|
|
• |
require product seizure or detention, recalls or refuse to permit the import or export of products. |
Any government investigation of alleged violations of law would require us to expend significant time and resources in response and could generate adverse publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to develop and commercialize our products and our value and our operating results would be adversely affected. In addition, regulatory authorities’ policies (such as those of the FDA or EMA) may change and additional government regulations may
43
be enacted that could prevent, limit or delay regulatory approval of our drug or biologic candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are otherwise not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, which would adversely affect our business, prospects and ability to achieve or sustain profitability.
Our commercial success will depend upon attaining significant market acceptance of our drug or biologic candidates, if approved, among physicians, patients, third-party payors and other members of the medical community.
Even if we obtain regulatory approval for our drug or biologic candidates, the approved products may nonetheless fail to gain sufficient market acceptance among physicians, third-party payors, patients and other members of the medical community, which is critical to commercial success. If an approved product does not achieve an adequate level of acceptance, we may not generate significant product revenues or any profits from operations. The degree of market acceptance of any drug or biologic candidate for which we receive approval depends on a number of factors, including:
|
|
• |
the efficacy and potential advantages compared to alternative treatments or competitive products; |
|
|
• |
perceptions by the medical community, physicians, and patients regarding the safety and effectiveness of our products and the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
|
|
• |
the size of the market for such drug or biologic candidate, based on the size of the patient subsets that we are targeting, in the territories for which we gain regulatory approval and have commercial rights; |
|
|
• |
the safety of the drug or biologic candidate as demonstrated through broad commercial distribution; |
|
|
• |
the ability to offer our drug or biologic candidates for sale at competitive prices; |
|
|
• |
the availability of adequate reimbursement and pricing for our products from governmental health programs and other third-party payors; |
|
|
• |
relative convenience and ease of administration compared to alternative treatments; |
|
|
• |
cost-effectiveness of our product relative to competing products; |
|
|
• |
the prevalence and severity of any side effects; |
|
|
• |
the adequacy of supply of our drug or biologic candidates; |
|
|
• |
the timing of any such marketing approval in relation to other product approvals; |
|
|
• |
any restrictions on concomitant use of other medications; |
|
|
• |
support from patient advocacy groups; and |
|
|
• |
the effectiveness of sales, marketing and distribution efforts by us and our licensees and distributors, if any. |
If our drug or biologic candidates are approved but fail to achieve an adequate level of acceptance by key market participants, we will not be able to generate significant revenues, and we may not become or remain profitable, which may require us to seek additional financing.
Our ability to negotiate, secure and maintain third-party coverage and reimbursement for our drug or biologic candidates may be affected by political, economic and regulatory developments in the United States, the European Union and other jurisdictions. Governments continue to impose cost containment measures, and third-party payors are increasingly challenging prices charged for medicines and examining their cost effectiveness, in addition to their safety and efficacy. These and other similar developments could significantly limit the degree of market acceptance of any drug or biologic candidate of ours that receives marketing approval in the future. See also the risk disclosures below under “Healthcare legislative reform measures may have a material adverse effect on our business, financial condition or results of operations.”
Healthcare legislative reform measures may have a material adverse effect on our business, financial condition or results of operations.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. For example, in March 2010, the Patient Protection and Affordable Care Act (the “ACA”), was passed. The ACA was a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of health care spending, enhance remedies against fraud
44
and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. The ACA also included the Biologics Price Competition and Innovation Act (the “BPCIA”), that created the abbreviated application and licensure pathway for biosimilar and interchangeable biological products. As another example, the 2021 Consolidated Appropriations Act, which was signed into law on December 27, 2020, incorporated extensive health care provisions and amendments to existing laws, including a requirement that all manufacturers of drugs and biological products covered under Medicare Part B report the product’s average sales price to the Department of Health and Human Services (“HHS”) beginning on January 1, 2022, as well as several changes to the statutes governing FDA’s drug and biologic programs.
Further legislative and regulatory changes under the ACA remain possible, although it is unknown what form any such future changes or any law would take, and how or whether it may affect the biopharmaceutical industry as a whole or our business in the future. We expect that changes or additions to the ACA, the Medicare and Medicaid programs, and changes stemming from other healthcare reform measures, especially with regard to healthcare access, financing or other legislation in individual states, could have a material adverse effect on the health care industry in the U.S.
In the United States and in some other jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the health care system that could prevent or delay marketing approval of our drug or biologic candidates, restrict or regulate post-approval activities, or affect our ability to profitably sell any drug or biologic candidates for which we obtain marketing approval, if any. For example, as part of the Consolidated Appropriations Act for 2023, Congress provided the FDA additional authorities related to the accelerated approval pathway for human drugs and biologics. Under these recent amendments to the FDCA, the agency may require a sponsor of a product granted accelerated approval to have a confirmatory trial underway prior to approval. The amendments also give the FDA the option of using expedited procedures to withdraw product approval if the sponsor’s confirmatory trial fails to verify the claimed clinical benefits of the product. Legislators continue to debate various reforms that have the potential to significantly alter FDA authorities or existing agency policies pertaining to biopharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals, if any, of our drug or biologic candidates, may be or whether such changes will have any other impacts on our business. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing conditions and other requirements.
Further, over the past several years there has been heightened governmental scrutiny over the manner in which biopharmaceutical manufacturers set prices for their marketed products, which has resulted in several U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. The probability of success of these newly announced policies, many of which have been or are expected to be subjected to legal challenge in the federal court system, and their potential impact on the U.S. prescription drug marketplace is unknown. There are likely to be continued political and legal challenges associated with implementing these reforms as they are currently envisioned. For example, on August 16, 2022, President Biden signed into the law the Inflation Reduction Act of 2022 (the “IRA”). Among other things, the IRA has multiple provisions that may impact the prices of drug products that are both sold into the Medicare program and throughout the United States. Starting in 2023, a manufacturer of drugs or biological products covered by Medicare Parts B or D must pay a rebate to the federal government if their drug product’s price increases faster than the rate of inflation. This calculation is made on a drug product by drug product basis and the amount of the rebate owed to the federal government is directly dependent on the volume of a drug product that is paid for by Medicare Parts B or D. Additionally, starting for payment year 2026, the Centers for Medicare & Medicaid Services (“CMS”) will negotiate drug prices annually for a select number of single source Part D drugs without generic or biosimilar competition. CMS will also negotiate drug prices for a select number of Part B drugs starting for payment year 2028. If a drug product is selected by CMS for negotiation, it is expected that the revenue generated from such drug will decrease. CMS has begun to implement these new authorities but their impact on the biopharmaceutical industry in the United States remains uncertain.
Additionally, in July 2021, President Biden issued a sweeping executive order promoting competition in the American economy that includes several mandates pertaining to the pharmaceutical and healthcare insurance industries. Among other things, the executive order directs the FDA to work towards implementing a system for importing drugs from Canada (following finalization of the Canadian drug importation rulemaking in October 2020), and to clarify and improve the standards for interchangeable biosimilars. The Biden order also called on HHS to release a comprehensive plan to combat high prescription drug prices, and it includes several directives regarding the Federal Trade Commission’s oversight of potentially anticompetitive practices within the pharmaceutical industry. The drug pricing plan released by HHS in September 2021 in response to the executive order makes clear that the Biden Administration supports aggressive action to address rising drug prices, and such actions have started within the implementation of the IRA. In addition to the IRA’s drug price negotiation provisions summarized above, President Biden’s Executive Order 14087, issued in October 2022, called for the CMS innovation center to prepare and submit a report to the White House on potential payment and delivery modes that would complement to IRA, lower drug costs, and promote access to innovative drugs. As of mid-January 2023, the report had not been released but it is expected to further inform the current Administration’s priorities and activities in this area.
45
Accordingly, there remains a large amount of uncertainty regarding the federal government’s approach to making pharmaceutical treatment costs more affordable for patients.
There also are a number of state and local legislative and regulatory efforts related to drug or biologic pricing, including drug or biologic price transparency laws that apply to pharmaceutical manufacturers, that may have an impact on our business. Individual states in the U.S. have become increasingly active in passing legislation and implementing regulations designed to control product pricing, including price or patient reimbursement constraints, discounts, and restrictions on certain product access. In December 2020, the U.S. Supreme Court held unanimously that federal law does not preempt the states’ ability to regulate pharmacy benefit managers (“PBMs”) and other members of the health care and pharmaceutical supply chain, an important decision that appears to be leading towards further and more aggressive efforts by states in this area. The Federal Trade Commission in mid-2022 also launched sweeping investigations into the practices of the PBM industry that could lead to additional federal and state legislative or regulatory proposals targeting such entities’ operations, pharmacy networks, or financial arrangements. Significant efforts to change the PBM industry as it currently exists in the U.S. may affect the entire pharmaceutical supply chain and the business of other stakeholders, including biopharmaceutical product developers like us.
In the European Union, similar political, economic and regulatory developments may affect our ability to profitably commercialize our products. In addition to continuing pressure on prices and cost containment measures, legislative developments at the European Union or EU member state level may result in significant additional requirements or obstacles that may increase our operating costs.
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action. We expect that additional federal and state health care reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for health care products and services, which could result in limited coverage and reimbursement and reduced demand for our products, once approved, or additional pricing pressures.
Our relationships with prescribers, purchasers, third-party payors and patients will be subject to applicable anti-kickback, fraud and abuse and other health care laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
If we obtain FDA approval for any of our drug or biologic candidates and begin commercializing those products in the United States, our operations will be subject to additional health care statutory and regulatory requirements and oversight by federal and state governments in the United States as well as foreign governments in the jurisdictions in which we conduct our business. Physicians, other health care providers and third-party payors will play a primary role in the recommendation, prescription and use of any drug or biologic candidates for which we obtain marketing approval. In the U.S., our future arrangements with such third parties may expose us to broadly applicable fraud and abuse and other health care laws and regulations that may constrain our business or financial arrangements and relationships through which we market, sell and distribute any products for which we may obtain marketing approval. Violations of the fraud and abuse laws are punishable by criminal and civil sanctions, including, in some instances, exclusion from participation in federal and state health care programs, including Medicare and Medicaid. Restrictions under applicable domestic and foreign health care laws and regulations include but are not limited to the following:
|
|
• |
the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase order or recommendation of a good or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
|
|
• |
federal civil and criminal false claims laws and civil monetary penalty laws, including the U.S. False Claims Act, which impose criminal and civil penalties against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; actions may be brought by the government or a whistleblower and may include an assertion that a claim for payment by federal health care programs for items and services which results from a violation of the federal Anti-Kickback Statue constitutes a false or fraudulent claim for purposes of the False Claims Act; |
46
|
|
|
|
• |
the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) that imposes criminal and civil liability for executing a scheme to defraud any health care benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for health care benefits, items or services; similar to the U.S. federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
|
|
• |
analogous state and foreign laws and regulations relating to health care fraud and abuse, such as state anti-kickback and false claims laws, that may apply to sales or marketing arrangements and claims involving health care items or services reimbursed by non-governmental third-party payors, including private insurers; |
|
|
• |
the federal transparency requirements, sometimes referred to as the “Sunshine Act,” enacted as part of the ACA, which requires, among other things, manufacturers of drugs, devices, biologics and medical supplies that are reimbursed under Medicare, Medicaid, or the Children’s Health Insurance Program to report annually to CMS information related to payments and other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain advanced non-physician health care practitioners (such as physician assistants and nurse practitioners) and teaching hospitals, as well as physician ownership and investment interests, including such ownership and investment interests held by a physician’s immediate family members; |
|
|
• |
analogous state and foreign laws that require pharmaceutical companies to track, report and disclose to the government and/or the public information related to payments, gifts, and other transfers of value or remuneration to physicians and other health care providers, marketing activities or expenditures, or product pricing or transparency information, or that require pharmaceutical companies to implement compliance programs that meet certain standards or to restrict or limit interactions between pharmaceutical manufacturers and members of the health care industry; |
|
|
• |
the U.S. federal laws that require pharmaceutical manufacturers to report certain calculated product prices to the government or provide certain discounts or rebates to government authorities or private entities, often as a condition of reimbursement under federal health care programs; |
|
|
• |
HIPAA, which imposes obligations on certain covered entity health care providers, health plans, and health care clearinghouses as well as their business associates that perform certain services involving the use or disclosure of individually identifiable health information, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; and |
|
|
• |
state and foreign laws that govern the privacy and security of health information in certain circumstances, including state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, many of which differ from each other in significant ways or conflict with each other and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable health care laws and regulations will involve substantial costs. If the FDA or a comparable foreign regulatory authority approves any of our drug or biologic candidates, we will be subject to an expanded number of these laws and regulations and will need to expend resources to develop and implement policies and processes to promote ongoing compliance. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other health care laws and regulations, resulting in government enforcement actions. If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil, criminal and administrative penalties, damages, fines, exclusion from participation in government health care programs, such as Medicare and Medicaid, imprisonment, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
We may be subject to, or may in the future become subject to, U.S. federal and state, and foreign laws and regulations imposing obligations on how we collect, use, disclose, store and process personal information. Our actual or perceived failure to comply with such obligations could result in liability or reputational harm and could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our potential future customer base, and thereby decrease our revenue.
In many activities, including the conduct of clinical trials, we may be subject to laws and regulations governing data privacy and the protection of health-related and other personal information. These laws and regulations govern our processing of personal data, including the collection, access, use, analysis, modification, storage, transfer, security breach notification, destruction and disposal of personal data.
47
The privacy and security of personally identifiable information stored, maintained, received or transmitted, including electronically, is subject to significant regulation in the United States and abroad. While we strive to comply with all applicable privacy and security laws and regulations, legal standards for privacy continue to evolve and any failure or perceived failure to comply may result in proceedings or actions against us by government entities, affected individuals or others, which could be extraordinarily expensive to defend and could cause reputational harm, which could have a material adverse effect on our business.
Numerous foreign, federal and state laws and regulations govern collection, dissemination, use and confidentiality of personally identifiable health information, including state privacy and confidentiality laws (including state laws requiring disclosure of breaches), federal and state consumer protection and employment laws, HIPAA and European and other foreign data protection laws. These laws and regulations are increasing in complexity and number and may change frequently and sometimes conflict. The European Union’s omnibus data protection law, the General Data Protection Regulation (“GDPR”) took effect on May 25, 2018. The GDPR imposes numerous requirements on entities that process personal data in the context of an establishment in the European Economic Area (“EEA”) or that process the personal data of data subjects who are located in the EEA. These requirements include, for example, establishing a basis for processing, providing notice to data subjects, developing procedures to vindicate expanded data subject rights, implementing appropriate technical and organizational measures to safeguard personal data, and complying with restrictions on the cross-border transfer of personal data from the EEA to countries that the European Union does not consider to have in place adequate data protection legislation, such as the United States. The GDPR additionally establishes heightened obligations for entities that process “special categories” of personal data, such as health data. Nearly all clinical trials involve the processing of these “special categories” of personal data, and thus processing of personal data collected during the course of clinical trials is subject to heightened protections under the GDPR. Violations of the GDPR can lead to penalties of up to €20 million or 4% of an entity’s annual turnover. The United Kingdom has incorporated the GDPR into its Data Protection Act 2018, and substantially equivalent requirements and penalties apply in the United Kingdom.
On July 16, 2020, the Court of Justice of the European Union (the “CJEU”) issued a landmark opinion in the case Maximilian Schrems vs. Facebook (Case C-311/18), called Schrems II. This decision calls into question certain data transfer mechanisms as between the European Union member states and the United States. The CJEU is the highest court in Europe and the Schrems II decision heightens the burden on data importers to assess U.S. national security laws on their business, and future actions of European Union data protection authorities are difficult to predict at this early date. Consequently, there is some risk of any such data transfers from the European Union being halted by one or more European Union member states. Any contractual arrangements requiring the transfer of personal data from the European Union to us in the United States will require greater scrutiny and assessments as required under Schrems II and may have an adverse impact on cross-border transfers of personal data or increase costs of compliance.
HIPAA establishes a set of national privacy and security standards for the protection of protected health information (“PHI”) by health plans, health care clearinghouses and health care providers that submit certain covered transactions electronically, or covered entities, and their “business associates,” which are persons or entities that perform certain services for, or on behalf of, a covered entity that involve creating, receiving, maintaining or transmitting PHI. While we are not currently a covered entity or business associate under HIPAA, we are indirectly impacted by HIPAA because HIPAA regulates the ability of clinical investigators and other health care providers to share PHI with us. Failure to receive this information properly could subject us or our health care provider collaborators to HIPAA’s criminal penalties, which may include fines up to $250,000 per violation and/or imprisonment. In addition, responding to government investigations regarding alleged violations of these and other laws and regulations, even if ultimately concluded with no findings of violations or no penalties imposed, can consume company resources and impact our business and, if public, harm our reputation.
In addition, to the federal privacy regulations, there are a number of state laws regarding the privacy and security of health information and personal data that are applicable to clinical laboratories. The compliance requirements of these laws, including additional breach reporting requirements, and the penalties for violation vary widely and new privacy and security laws in this area are evolving. For example, several states, such as California, have implemented comprehensive privacy laws and regulations. The California Confidentiality of Medical Information Act (“CMIA”) imposes restrictive requirements regulating the use and disclosure of health information and other personally identifiable information. In addition to fines and penalties imposed upon violators, some of these state laws also afford private rights of action to individuals who believe their personal information has been misused. California’s patient privacy laws, for example, provide for penalties of up to $250,000 and permit injured parties to sue for damages. In addition to the CMIA, California also recently enacted the California Consumer Privacy Act of 2018 (“CCPA”) which became effective January 1, 2020. The CCPA, among other things, creates new data privacy obligations for covered businesses, and provides new privacy rights for California residents, including the right to opt out of certain disclosures of their information. It also creates new privacy rights for California residents and increases the privacy and security obligations of entities handling personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches, which is expected to increase data breach litigation. Although the law includes limited exceptions, including for PHI maintained by a covered entity or business associate under HIPAA and medical information maintained by healthcare providers under the CMIA, it may regulate or impact our processing of personal information depending on the context. Further, the California Privacy Rights Act (“CPRA”) went into effect on January 1, 2023, amending the CCPA. The CPRA imposes additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain
48
uses of sensitive data. It also created a new California Privacy Protection Agency authorized to issue substantive regulations and is expected to result in increased privacy and information security enforcement. The CPRA also extends the provisions of both the CCPA and the CPRA to the personal information of California-based employees. In addition to California, more U.S. states are enacting similar legislation, increasing compliance complexity and increasing risks of failures to comply. In 2023, comprehensive privacy laws in Virginia, Colorado, Connecticut, and Utah will all take effect.
As various states, such as California, Virginia, Colorado, Connecticut, and Utah implement their own privacy laws and regulations, the interplay of federal and state laws may be subject to varying interpretations by courts and government agencies, creating complex compliance issues for us and potentially exposing us to additional expense, adverse publicity and liability. Further, as regulatory focus on privacy issues continues to increase and laws and regulations concerning the protection of personal information expand and become more complex, these potential risks to our business could intensify. The legislative and regulatory landscape for privacy and data security continues to evolve, and there has been an increasing focus on privacy and data security issues which may affect our business. Failure to comply with current and future laws and regulations could result in government enforcement actions (including the imposition of significant penalties), criminal and/or civil liability for us and our officers and directors, private litigation and/or adverse publicity that negatively affects our business.
Reliance on government funding for our programs may add uncertainty to our research and commercialization efforts with respect to those programs that are tied to such funding and may impose requirements that limit our ability to take certain actions, increase the costs of commercialization and production of drug or biologic candidates developed under those programs and subject us to potential financial penalties, which could materially and adversely affect our business, financial condition and results of operations.
During the course of our development of our drug or biologic candidates, we have been funded in significant part through state grants, including but not limited to the substantial funding we have received from the Cancer Prevention & Research Institute of Texas (“CPRIT”). On September 18, 2018, we entered into our second CPRIT award grant contract for our CD38 targeted ETB program (“the CD38 CPRIT Agreement”), which was extended in September 2022. In addition to the funding we have received to date, we have applied and intend to continue to apply for federal and state grants to receive additional funding in the future, which may or may not be successful. Contracts and grants funded by the U.S. government, state governments and their related agencies, including our contracts with the State of Texas pertaining to funds we have already received, include provisions that reflect the government’s substantial rights and remedies, many of which are not typically found in commercial contracts, including powers of the government to:
|
|
• |
require repayment of all or a portion of the grant proceeds, in certain cases with interest, in the event we violate certain covenants pertaining to various matters that include any potential relocation outside of the State of Texas, failure to achieve certain milestones or to comply with terms relating to use of grant proceeds, or failure to comply with certain laws; |
|
|
• |
terminate agreements, in whole or in part, for any reason or no reason; |
|
|
• |
reduce or modify the government’s obligations under such agreements without the consent of the other party; |
|
|
• |
claim rights, including march-in and other intellectual property rights, in products and data developed under such agreements; |
|
|
• |
audit contract-related costs and fees, including allocated indirect costs; |
|
|
• |
suspend the contractor or grantee from receiving new contracts pending resolution of alleged violations of procurement laws or regulations; |
|
|
• |
impose the State of Texas or U.S. manufacturing requirements for products that embody inventions conceived or first reduced to practice under such agreements; |
|
|
• |
impose the qualifications for the engagement of manufacturers, suppliers and other contractors as well as other criteria for reimbursements; |
|
|
• |
suspend or debar the contractor or grantee from doing future business with the government; |
|
|
• |
control and potentially prohibit the export of products; |
49
|
|
• |
pursue criminal or civil remedies under the False Claims Act, False Statements Act and similar remedy provisions specific to government agreements; and |
|
|
• |
limit the government’s financial liability to amounts appropriated by the State of Texas on a fiscal-year basis, thereby leaving some uncertainty about the future availability of funding for a program even after it has been funded for an initial period. |
In addition to those powers set forth above, the government funding we may receive could also impose requirements to make payments based upon sales of our products in the future. For example, under the terms of our CD38 CPRIT Agreement, we are required to pay CPRIT a percentage of our revenues from sales of products directly funded by CPRIT, or received from our licensees or sub licensees, at a percentage in the low to mid-single digits until the aggregate amount of such payments equals 400% of the funds we receive from CPRIT, and thereafter at a rate of one-half percent.
We may not have the right to prohibit the State of Texas or, if relevant under possible future federal grants, the U.S. government, from using certain technologies developed by us, and we may not be able to prohibit third-party companies, including our competitors, from using those technologies in providing products and services to the U.S. government. The U.S. government generally takes the position that it has the right to royalty-free use of technologies that are developed under U.S. government contracts. These and other provisions of government grants may also apply to intellectual property we license now or in the future.
In addition, government contracts and grants normally contain additional requirements that may increase our costs of doing business, reduce our profits and expose us to liability for failure to comply with these requirements. These requirements include, for example:
|
|
• |
specialized accounting systems unique to government contracts and grants; |
|
|
• |
mandatory financial audits and potential liability for price adjustments or recoupment of government funds after such funds have been spent; |
|
|
• |
public disclosures of certain contract and grant information, which may enable competitors to gain insights into our research program; and |
|
|
• |
mandatory socioeconomic compliance requirements, including labor standards, non-discrimination and affirmative action programs and environmental compliance requirements. |
If we fail to maintain compliance with any such requirements that may apply to us now or in the future, we may be subject to potential liability and to termination of our contracts.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on our business, financial condition or results of operations.
Our research and development activities and our third-party manufacturers’ and suppliers’ activities involve the controlled storage, use, and disposal of hazardous materials, including the components of our drug or biologic candidates and other hazardous compounds. We and our manufacturers and suppliers are subject to laws and regulations governing the use, manufacture, storage, handling, and disposal of these hazardous materials. In some cases, these hazardous materials and various wastes resulting from their use are stored at our and our manufacturers’ facilities pending their use and disposal. We cannot eliminate the risk of contamination, which could cause an interruption of our commercialization efforts, research and development efforts and business operations; environmental damage resulting in costly clean-up; and liabilities under applicable laws and regulations governing the use, storage, handling, and disposal of these materials and specified waste products. Although we believe that the safety procedures utilized by us and our third-party manufacturers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable for any resulting damages and such liability could exceed our resources and state or federal or other applicable authorities may curtail our use of specified materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex, change frequently, and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance. We do not currently carry biological or hazardous waste insurance coverage.
50
Inadequate funding for the FDA, the SEC and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent our drug or biologic candidates from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs and biologic products to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, the U.S. government has shut down several times, including from December 22, 2018 through January 25, 2019, and congressional impasses periodically threaten to cause future government shutdowns. When a shutdown occurs, certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities. Moreover, government shutdowns or slowdowns, such as those caused recently by the federal response to the COVID-19 pandemic and that could occur again in the event of another public health or other national emergency, can increase the time needed for an agency to complete its review or make final approvals or other administrative decisions. If a prolonged government shutdown or slowdown occurs, it could significantly affect the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, in our operations as a public company, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Risks Related to Our Intellectual Property
Our ability to compete effectively may decline if we are unable to establish intellectual property rights or if our intellectual property rights are inadequate to protect our ETB technology, present and future drug or biologic candidates and related processes for our developmental pipeline.
We rely or will rely upon a combination of patents, trade secret protection, and confidentiality agreements to protect our intellectual property related to our technologies and drug or biologic candidates. Our commercial success and viability depend in large part on our current and potential future licensors or collaboration partners’ ability to obtain, maintain and enforce patent and other intellectual property protections in the United States, Europe and other countries worldwide with respect to our current and future proprietary technologies and drug or biologic candidates. If we or our current or future licensors or collaboration partners do not adequately protect such intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could materially harm our business, negatively affect our position in the marketplace, limit our ability to commercialize drug or biologic candidates and delay or render impossible our achievement of profitability.
Our strategy and future prospects are based, in part, on our patent portfolio. We and our current and future licensors or collaboration partners or licensees will best be able to protect our proprietary ETB technologies, drug or biologic candidates and their uses from unauthorized use by third parties to the extent that valid and enforceable patents, other regulatory exclusivities or effectively protected trade secrets, cover them. We have sought to protect our proprietary position by filing in the United States and elsewhere patent applications related to our proprietary ETB technologies, drug or biologic candidates and methods of use that are important to our business. This process is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain meaningful patent protection.
Intellectual property rights have limitations and do not necessarily address all potential threats to our competitive advantage. Our ability to obtain patent protection for our proprietary technologies, drug or biologic candidates and their uses is uncertain, and the degree of future protection afforded by our intellectual property rights is uncertain due to a number of factors, including, but not limited to:
|
|
• |
we or our past, current or future licensors or collaboration partners may not have been the first to make the inventions disclosed in or covered by pending patent applications or issued patents; |
|
|
• |
we or our past, current or future licensors or collaboration partners may not have been the first to file patent applications, including covering our ETB technology, drug or biologic candidates, compositions or their uses; |
|
|
• |
others may independently develop identical, similar or alternative methods, products, drug or biologic candidates or compositions and uses thereof; |
|
|
• |
we or our past, current or future licensors or collaboration partners’ disclosures in patent applications may not be sufficient to meet the statutory requirements for patentability; |
51
|
|
|
|
• |
any or all of our or our current or future licensors or collaboration partners’ pending patent applications may not result in issued patents; |
|
|
• |
we or our current or future licensors or collaboration partners may not seek or obtain patent protection in jurisdictions or countries that may provide us with a significant business opportunity; |
|
|
• |
we or our current or future licensors or collaboration partners might seek or obtain patent protection in jurisdictions or countries that might not provide us with a significant business opportunity; |
|
|
• |
any patents issued to us or to our past, current or future licensors or collaboration partners, or to us and to our past, current or future licensors or collaboration partners, may not provide a basis for commercially viable products, may not provide any competitive advantages or may be successfully challenged by one or more third parties; |
|
|
• |
we or our past, current or future licensors’ or collaboration partners’ products, drug or biologic candidates, compositions, methods or uses thereof may not be patentable; |
|
|
• |
we or our past, current or future licensors or collaboration partners might fail to maintain our or their patents, resulting in their abandonment; |
|
|
• |
we or our current or future licensors or collaboration partners might fail to obtain patent term extensions available in the United States or in foreign jurisdictions or countries; |
|
|
• |
others may design around our or our past, current or future licensors’ or collaboration partners’ patent claims to produce competitive technologies, products or uses which fall outside of the scope of our patents or other intellectual property rights; |
|
|
• |
others may identify prior art or other bases which could render unpatentable our or our past, current or future licensors’ or collaboration partners’ patent applications, or invalidate our or our past, current or future licensors or collaboration partners’ patents; |
|
|
• |
our competitors might conduct research and development activities in the United States and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we or our past, current or future licensors or collaboration partners do not have patent rights, and then use the information learned from such activities to develop competitive products for sale in major commercial markets; or |
|
|
• |
we or our current or future licensors or collaboration partners may not develop additional proprietary technologies or products that are patentable. |
Further, the patent position of biotechnology and pharmaceutical companies generally is highly uncertain and involves complex legal and factual questions for which legal principles remain unsettled. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our or our competitors’ drug or biologic candidates or their uses in the United States or in other countries. There is no assurance that all potentially relevant prior art relating to our patents and patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue, and even if such patents cover our technologies, drug or biologic candidates, compositions or their uses, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed, found unenforceable or invalidated. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our drug or biologic candidates or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
We, independently or together with our collaboration partners, have filed patent applications covering various aspects of our ETB technology, drug or biologic candidates and associated assays and uses. We cannot offer any assurances about which, if any, patents will issue, the breadth of any such patent or whether any issued patents will be found invalid and unenforceable or will be threatened by one or more third parties. Any successful opposition or challenge to these patents or to any other patents owned by or licensed to us after patent issuance could deprive us of rights necessary for the successful commercialization of any drug or biologic candidates that we may develop. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a drug or biologic candidate under patent protection could be reduced.
If we cannot obtain and maintain effective protection of exclusivity from our regulatory efforts and intellectual property rights, including patent protection or data or market exclusivity for our technologies, drug or biologic candidates, compositions or their uses, we may not be able to compete effectively, and our business and results of operations would be harmed.
52
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on drug or biologic candidates in all countries throughout the world would be prohibitively expensive. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal or state laws in the United States. Competitors may use our technologies to develop our own products in jurisdictions where we have not obtained patent protection and may also export infringing products to territories where we do not have patent protection, or to territories where we have patent protection, but where enforcement is not as strong as that in the United States. These products may compete with our products, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of some countries, particularly some developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to healthcare, medicine, or biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our resources, efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
We may not have sufficient patent term or regulatory exclusivity protections for our drug or biologic candidates to effectively protect our competitive position.
Patents have a limited term. In the United States and most jurisdictions worldwide, the statutory expiration of a non-provisional patent is generally 20 years after it is first filed. Although various extensions may be available, the life of a patent, and the protection it affords, is limited. Even if patents covering our technologies, drug or biologic candidates and associated uses are obtained, once the patent’s life has expired, including for failure to pay maintenance fees or annuities, we may be open to competition from generic, biosimilar or biobetter medications.
Patent term extensions under the Hatch-Waxman Act in the United States, and regulatory extensions in Japan and certain other countries, and under Supplementary Protection Certificates in Europe, may be available to extend the patent or market or data exclusivity terms of our drug or biologic candidates depending on the timing and duration of the regulatory review process relative to patent term. In addition, upon issuance of a United States patent, any patent term may be adjusted based on specified delays during patent prosecution caused by the applicant(s) or the United States Patent and Trademark Office (the “USPTO”). Although we will likely seek patent term extensions in the U.S. and in one or more foreign jurisdictions where available, we cannot provide any assurances that any such patent term extensions will be granted and, if so, for how long. As a result, we may not be able to maintain exclusivity for our drug or biologic candidates for an extended period after regulatory approval, if any, which would negatively impact our business, financial condition, results of operations and prospects. If we do not have sufficient patent term or regulatory exclusivity to protect our drug or biologic candidates, our business and results of operations will be adversely affected.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our technologies and products, and recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
As is the case with other biotechnology companies, our success is heavily dependent on patents. Obtaining and enforcing patents in the biotechnology industry involve both technological and legal complexity, and is therefore costly, time-consuming and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in specified circumstances and weakened the rights of patent owners in specified situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
On September 16, 2011, the Leahy-Smith America Invents Act (“AIA”) was signed into law. Under the AIA, as of March 16, 2013, the United States transitioned to a “first-inventor-to-file” system for deciding which party should be granted a patent when two or more patent applications are filed by different parties claiming the same invention. A third party that filed or files a patent application with the USPTO after March 16, 2013 but before we file an application could therefore be granted a patent covering an invention of ours even if we had made the invention before it was made by the third party. Since patent applications in the United States and most other countries are confidential at least 18 months after filing, we cannot be certain that we were the first to file any patent application related to our drug or biologic candidates.
The AIA also provides a process known as inter partes review (“IPR”), which has been used by many third parties to challenge and invalidate patents. The IPR process is not limited to patents filed after the AIA was enacted and would therefore be available to a third party seeking to invalidate any of our U.S. patents, even those issued or filed before March 16, 2013. Because of a lower
53
evidentiary standard in USPTO proceedings compared to the evidentiary standard in U.S. federal court necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures, e.g., an IPR, to invalidate our patent claims that would not have been invalidated if first challenged by the third party in a district court action.
We could be required to incur significant expenses to obtain our intellectual property rights, and we cannot ensure that we will obtain meaningful patent protection for our drug or biologic candidates.
The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. In addition, it is also possible that we will fail to identify patentable aspects of further inventions made in the course of our research, development or commercialization activities before they are publicly disclosed, making it in many cases too late to obtain patent protection on them. Further, given the amount of time required for the development, testing and regulatory review of new drug or biologic candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. We expect to seek extensions of patent terms where these are available in any countries where we are prosecuting patents. This includes in the United States under the Drug Price Competition and Patent Term Restoration Act of 1984, which permits a patent term extension of up to five years beyond the expiration of a patent that covers an approved product where the permission for the commercial marketing or use of the product is the first permitted commercial marketing or use, and as long as the remaining term of the patent does not exceed 14 years. However, the applicable authorities, including the FDA in the United States, and any comparable regulatory authority in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or may grant more limited extensions than we request. If this occurs, our competitors may be able to take advantage of our investment in development and clinical trials by referencing our clinical and preclinical data and launch their product earlier than might otherwise be the case. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the United States, and these foreign laws may also be subject to change. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing or in some cases not at all. Therefore, we cannot be certain that we, our past, current or future collaboration partners or licensors were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we, our past, current or future collaboration partners or licensors were the first to file for patent protection of such inventions.
Issued patents covering our ETB technologies, drug or biologic candidates, compositions or uses could be found invalid or unenforceable if challenged in a patent office or court.
Even if our past, current or future collaboration partners’ or licensors’ patents do successfully issue and even if such patents cover our technologies, drug or biologic candidates, compositions or methods of use, third parties may initiate interference, re-examination, post-grant review, IPR or derivation actions in the USPTO; may initiate third party oppositions in the European Patent Office (“EPO”); or may initiate similar actions challenging the validity, enforceability, scope or term of such patents in other patent administrative or court proceedings worldwide, which may result in patent claims being narrowed or invalidated. Such proceedings could result in revocation or amendment of our patents in such a way that they no longer cover competitive technologies, drug or biologic candidates, compositions or methods of use. Further, if we initiate legal proceedings against a third party to enforce a patent covering our technologies, drug or biologic candidates, compositions or uses, the defendant could counterclaim that our relevant patent is invalid or unenforceable. In patent litigation in the United States, certain European and other countries worldwide, it is commonplace for defendants to make counterclaims alleging invalidity and unenforceability in the same proceeding, or to commence parallel defensive proceedings such as patent nullity actions to challenge validity and enforceability of asserted patent claims. Further, in the United States, a third party, including a licensee of one of our past, current or future collaboration partners’ patents, may initiate legal proceedings against us in which the third party challenges the validity, enforceability, or scope of our patent(s).
In administrative and court actions, grounds for a patent validity challenge may include alleged failures to meet any of several statutory requirements, including novelty, nonobviousness (or inventive step), clarity, adequate written description and enablement of the claimed invention. Grounds for unenforceability assertions include allegations that someone associated with the filing or prosecution of the patent withheld material information from the Examiner during prosecution in the USPTO or made a misleading statement during prosecution in the USPTO, the EPO or elsewhere. Third parties also may raise similar claims before administrative bodies in the USPTO or the EPO, even outside the context of litigation. The outcome following legal assertions of invalidity or unenforceability are unpredictable. With respect to patent claim validity, for example, we cannot be certain that there is no invalidating prior art, of which we or the patent examiner was unaware during prosecution. Further, we cannot be certain that all of the potentially relevant art relating to our patents and patent applications has been brought to the attention of every patent office. If a defendant or other patent challenger were to prevail on a legal assertion of invalidity or unenforceability, we could lose at least part, and perhaps all, of the patent protection on our ETB technology, drug or biologic candidates, compositions and associated uses.
54
We may become involved in lawsuits to protect or enforce our patents or other intellectual property rights, which could be expensive, time consuming and unsuccessful and have a material adverse effect on the success of our business.
Competitors may infringe our patents or the patents of any of our past, current or future licensors. If we or one of our past, current or future collaboration partners were to initiate legal proceedings against a third party to enforce a patent covering one of our drug or biologic candidates, the defendant could counterclaim that the patent covering our drug or biologic candidate is invalid and/or unenforceable. In addition, a third party might initiate legal proceedings against us alleging that our patent covering one or more of our drug or biologic candidates is invalid and/or unenforceable. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including novelty, nonobviousness, adequate written description, clarity or enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability is unpredictable.
There is also a risk that, even if the validity of such patents is upheld, the court will construe the patent’s claims narrowly, for example, such that they do not cover our drug or biologic candidates or decide that we do not have the right to stop the other party from using the claimed invention at issue on the grounds that our or our past, current or future collaboration partners’ patent claims do not cover the claimed invention. Third parties may in the future make claims challenging the inventorship or ownership of our intellectual property. An adverse outcome in a litigation or proceeding involving one or more of our patents could limit our ability to assert those patents against those parties or other competitors and may curtail or preclude our ability to exclude third parties from making and selling similar or competitive products.
Even if we were to establish infringement of our patent rights by a third party, the court may decide not to grant an injunction against further infringing activity and instead award only monetary damages, which may or may not be an adequate remedy. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could adversely affect the market price of our common stock. Moreover, there can be no assurance that we will have sufficient financial or other resources to file, pursue or maintain such infringement claims, which typically last for years before they are concluded and can involve substantial expenses. Even if we ultimately prevail in such claims, the monetary cost of such litigation and the diversion of the attention of our management and scientific personnel could outweigh any benefit we receive as a result of the proceedings.
Interference or derivation proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority or inventorship of inventions with respect to our patents or patent applications or those of any of our future licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation, interference proceedings, or derivation proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated with litigation and administrative proceedings could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our research programs, license necessary technology from third parties or enter into development partnerships that would help us bring our drug or biologic candidates to market.
If we are unable to protect the confidentiality of our trade secrets and know-how for our drug or biologic candidates or any future drug or biologic candidates, we may not be able to compete effectively in our proposed markets.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce and any other elements of our drug or biologic candidate discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors, contractors and other third parties. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors.
Although our current employment contracts require assignment of inventor’s rights of intellectual property to us, and we expect all of our employees and consultants to assign their inventions to us, and although all of our employees, consultants, advisors, and any third parties who have access to our proprietary know-how, information or technology are expected to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed or that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized disclosure of our trade secrets could impair our competitive position and may have a material adverse effect on our business, financial condition or results of operations. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating trade secrets.
55
Third party claims of intellectual property infringement could result in costly litigation or other proceedings and may prevent or delay our development and commercialization efforts.
Our research and development activities and commercial success depends in part on our ability to develop, manufacture, market and sell our drug or biologic candidates and use our proprietary technology without infringing the patent rights of third parties. Third parties may assert that we are employing their proprietary technology without authorization. We are currently not aware of U.S. or foreign patents or pending patent applications that are owned by one or more third parties and that cover our ETB drug or biologic candidates or therapeutic uses of those ETB drug or biologic candidates. In the future, we may identify such third-party U.S. and non-U.S. issued patents and pending applications. If we identify any such patents or pending applications, we may in the future pursue available proceedings in the U.S. and foreign patent offices to challenge the validity of these patents and patent applications. In addition, or alternatively, we may consider whether to seek to negotiate a license of rights to technology covered by one or more of such patents and patent applications. If any patents or patent applications cover our drug or biologic candidates or technologies or a requisite manufacturing process, we may not be free to manufacture or market our drug or biologic candidates, including MT-6402, MT-8421 or MT-0169, as planned, absent such a license, which may not be available to us on commercially reasonable terms, or at all.
It is also possible that we have failed to identify relevant third-party patents or applications. For example, patent applications filed before November 29, 2000 and patent applications filed after that date, but that will not be filed outside the United States, remain confidential until the patent applications issue as patents. Moreover, it is difficult for industry participants, including us, to identify all third-party patent rights that may be relevant to drug or biologic candidates and technologies with certainty. We may fail to identify relevant patents or patent applications or may identify pending patent applications of potential interest but incorrectly predict the likelihood that such patent applications may issue with claims of relevance to our technology. In addition, we may be unaware of one or more issued patents that would be infringed by the manufacture, sale or use of a current or future drug or biologic candidate, or we may incorrectly conclude that a patent office or court would determine that a third-party patent is invalid, unenforceable or not infringed by our activities. Additionally, pending patent applications that have been published can, subject to specified limitations, be later amended in a manner that could cover our technologies, our drug or biologic candidates or the use of our drug or biologic candidates.
There have been many lawsuits and other proceedings involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions and reexamination proceedings before the USPTO and corresponding foreign patent offices. Third parties own numerous U.S. and foreign issued patents and pending patent applications in the fields in which we are developing drug or biologic candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our drug or biologic candidates may be subject to claims of infringement of the patent rights of third parties. Parties making patent infringement claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our drug or biologic candidates. Defense of these claims, regardless of their merit, may involve substantial litigation expense and may require a substantial diversion of resources from our business. In the event of a successful claim of patent infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure. Further, if we were to seek a license from the third-party holder of any applicable intellectual property rights, we may not be able to obtain the applicable license rights when needed or on reasonable terms, or at all. Some of our competitors may be able to sustain the costs of complex patent litigation or proceeding more effectively than us due to their substantially greater resources. The occurrence of any of the above events could prevent us from continuing to develop and commercialize one or more of our drug or biologic candidates and our business could materially suffer.
We may be unsuccessful in obtaining or maintaining third-party intellectual property rights necessary to develop our ETB technologies or to commercialize our drug or biologic candidates and associated methods of use through acquisitions and in-licenses.
Presently, we have intellectual property rights to our ETB technologies under patent applications that we own and to certain targeting antibody domains through our license agreements. Because our programs may involve a range of ETB targets and antibody domains, which in the future may include targets and antibody domains that require the use of proprietary rights held by third parties, the growth of our business may likely depend in part on our ability to acquire, in-license or use these proprietary rights. In addition, our drug or biologic candidates may require specific formulations or manufacturing technologies to be safe, work effectively or be manufactured efficiently, and these rights may be held by others. We may be unable to acquire or in-license on reasonable terms any compositions, methods of use, processes or other third-party intellectual property rights from third parties that we identify. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities.
For example, we have previously collaborated, and may continue to collaborate, with federal, state or international academic institutions to accelerate our preclinical research or development under written agreements with these institutions. Typically, these institutions grant the rights to the collaborator and retain a non-commercial license to all rights as well as retain march-in rights in the situation that the collaborator fails to exercise or commercialize certain covered technologies. Regardless of such initial rights, we may be unable to exercise or commercialize certain funded technologies thereby triggering march-in rights of the funding institution. If we
56
are unable to do so, the institution may offer the intellectual property rights to other parties, potentially blocking our ability to pursue our program.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us, and vice versa. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to third-party intellectual property rights, our business, financial condition and prospects for growth could suffer.
If we are unable to successfully obtain and maintain rights to required third-party intellectual property, we may have to abandon development of that drug or biologic candidate or pay additional amounts to the third party, and our business and financial condition could suffer.
The patent protection and patent prosecution for some of our drug or biologic candidates may in the future be dependent on third parties.
While we normally seek to gain the right to fully prosecute the patent applications relating to our drug or biologic candidates, there may be times when certain patents or patent applications relating to our drug or biologic candidates, their compositions, uses or their manufacture may be controlled by our current or future collaboration partners or licensors. If any of our current or future collaboration partners fail to appropriately or broadly prosecute patent applications or maintain patent protection of claims covering any of our drug or biologic candidates, their compositions, uses or their manufacture, our ability to develop and commercialize those drug or biologic candidates may be adversely affected and we may not be able to prevent competitors from making, using, importing, offering to sell or selling competing products. In addition, even where we now have the right to control patent prosecution of patent applications or the maintenance of patents, we have licensed from third parties, presently or in the future, we may still be adversely affected or prejudiced by actions or inactions of our licensors in effect from actions prior to us assuming control over patent prosecution.
If we fail to comply with obligations in the agreements under which we license intellectual property and other rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose license rights that are important to our business.
We are and will continue to be a party to a number of intellectual property license collaboration and supply agreements that may be important to our business and expect to enter into additional license and supply agreements in the future. Our existing agreements impose, and we expect that future agreements will impose, various diligence, milestone payment, royalty, purchasing and other obligations on us. If we fail to comply with our obligations under these agreements, or if we are subject to a bankruptcy, our agreements may be subject to termination by the licensor, supplier, or other contract party, in which event we would not be able to develop, manufacture or market products covered by the license or subject to supply commitments.
We may be subject to claims that our employees, consultants or independent contractors wrongfully used or disclosed alleged confidential information of third parties or that our employees wrongfully used or disclosed alleged trade secrets of their former employers.
We employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including potential competitors. Although we have written agreements with these individuals, and although we make every effort to ensure that our employees, consultants and independent contractors do not use the proprietary information or intellectual property rights of others in their work for us, we may in the future be subject to claims that our employees, consultants or independent contractors wrongfully used or disclosed confidential information of third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful at defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Obtaining and maintaining our patent protection depends on compliance with various procedural, documentary, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to the USPTO or to foreign patent agencies in several stages over the lifetime of the patent, and periodic annuities are due to be paid for foreign patent applications in some foreign patent offices. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other requirements during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we or our current or future licensors or collaboration partners fail to maintain the patents and patent
57
applications covering our drug or biologic candidates, our competitors might be able to enter the market, which would have a material adverse effect on our business.
Our actual or perceived failures to comply with applicable data protection laws and regulations, and the increasing use of social media, could lead to government enforcement actions, private litigation and/or adverse publicity and could negatively affect our operating results and business.
We are subject to data protection laws and regulations that address privacy and data security. The legislative and regulatory landscape for data protection continues to evolve, and in recent years there has been an increasing focus on privacy and data security issues. In the United States, numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws and federal and state consumer protection laws govern the collection, use, disclosure and protection of health-related and other personal information. See the risk disclosures above under “We may be subject to, or may in the future become subject to, U.S. federal and state, and foreign laws and regulations imposing obligations on how we collect, use, disclose, store and process personal information. Our actual or perceived failure to comply with such obligations could result in liability or reputational harm and could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our potential future customer base, and thereby decrease our revenue.” Failure to comply with data protection laws and regulations could result in government enforcement actions, which could include civil or criminal penalties, private litigation and/or adverse publicity and could negatively affect our operating results and business. Complying with the enhanced obligations imposed by applicable international and U.S. privacy laws and regulations may result in significant costs to our business and require us to amend certain of our business practices. Further, enforcement actions and investigations by regulatory authorities related to data security incidents and privacy violations continue to increase. The future enactment of more restrictive laws, rules or regulations and/or future enforcement actions or investigations could have a materially adverse impact on us through increased costs or restrictions on our businesses, and non-compliance could result in regulatory penalties and significant legal liability.
Additionally, despite our efforts to monitor evolving social media communication guidelines and comply with applicable rules, there is risk that the use of social media by us or our employees to communicate about our drug or biologic candidates or business may cause us to be found in violation of applicable requirements, including but not limited to FDA prohibitions on the promotion of unapproved medical products. In addition, our employees may knowingly or inadvertently make use of social media in ways that may not comply with our internal policies or other legal or contractual requirements, which may give rise to liability, lead to the loss of trade secrets or other intellectual property, or result in public exposure of personal information of our employees, clinical trial patients, future customers and others. Our potential patient population may also be active on social media and use these platforms to comment on the perceived effectiveness of, or adverse experiences with, our drug or biologic candidates. Negative posts or comments about us or our drug or biologic candidates on social media could seriously damage our reputation, brand image and goodwill.
Risks Related to Our Reliance on Third Parties
We rely on third parties to conduct our clinical trials, manufacture our drug or biologic candidates and perform other services. If these third parties do not successfully carry out their contractual duties, meet expected timelines or otherwise conduct the trials as required or perform and comply with regulatory requirements, we may not be able to successfully complete clinical development, obtain regulatory approval or commercialize our drug or biologic candidates when expected or at all, and our business could be substantially harmed.
We have relied upon and plan to continue to rely upon third-party CROs to conduct, monitor and manage our ongoing clinical programs. We rely on these parties for execution of clinical trials and we manage and control only some aspects of their activities. We remain responsible for ensuring that each of our trials is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on the CROs does not relieve us of our regulatory responsibilities. We and our CROs and other vendors are required to comply with all applicable laws, regulations and guidelines, including those required by the FDA and comparable foreign regulatory authorities for all of our drug or biologic candidates in clinical development. If we, or any of our CROs or vendors, fail to comply with applicable laws, regulations or guidelines, the results generated in our clinical trials may be deemed unreliable, and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot be assured that our CROs or other vendors will meet these requirements, or that upon inspection by any regulatory authority, such regulatory authority will determine that efforts, including any of our clinical trials, comply with applicable requirements. Our failure to comply with these laws, regulations or guidelines may require us to repeat clinical trials, which would be costly and delay the regulatory approval process.
If any of our relationships with these third-party CROs terminates, we may not be able to enter into arrangements with alternative CROs in a timely manner or do so on commercially reasonable terms. In addition, our CROs may not prioritize our clinical trials relative to those of other customers, and any turnover in personnel or delays in the allocation of CRO employees by the CRO may negatively affect our clinical trials. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, including as a result of the COVID-19 pandemic, our clinical trials may be delayed or terminated, and we may not be able to meet our current plans with respect to our drug or biologic candidates. CROs also may involve higher costs than anticipated, which could negatively affect our financial condition and operations.
We currently have a cGMP manufacturing facility and we have developed the capability to manufacture drug or biologic candidates for use in the conduct of our clinical trials. We may not be able to manufacture drug or biologic candidates or there may be substantial technical or logistical challenges to supporting manufacturing demand for drug or biologic candidates. We may also fail to comply with cGMP requirements and standards which would require us to not utilize the manufacturing facility to make clinical trial
58
supply. We plan to rely at least in part on third-party contract manufacturers, and their responsibilities often include purchasing from third-party suppliers the materials necessary to produce our drug or biologic candidates for our clinical trials and to support future regulatory approval. We expect there to be a limited number of suppliers for some of the raw materials that we expect to use to manufacture our drug or biologic candidates, and we may not be able to identify alternative suppliers to prevent a possible disruption of the manufacture of our drug or biologic candidates for our clinical trials, and, if approved, ultimately for commercial sale.
Although we generally do not expect to begin a clinical trial unless we believe we have a sufficient supply of a drug or biologic candidate to complete the trial, any significant delay or discontinuity in the supply of a drug or biologic candidate, or the raw materials or other material components in the manufacture of the drug or biologic candidate, could delay completion of our clinical trials and potential timing for regulatory approval of our drug or biologic candidates, which would harm our business and results of operations. We do not yet have sufficient information to reliably estimate the cost of the commercial manufacturing of our drug or biologic candidates and our current costs to manufacture our drug or biologic candidates may not be commercially feasible, and the actual cost to manufacture our drug or biologic candidates could materially and adversely affect the commercial viability of our drug or biologic candidates. As a result, we may never be able to develop a commercially viable product.
In addition, our reliance on third-party manufacturers exposes us to the following additional risks:
|
|
• |
we may be unable to identify manufacturers to manufacture our drug or biologic candidates on acceptable terms or at all, because the number of qualified potential manufacturers is limited. Following NDA or BLA approval, a change in the manufacturing site could require additional approval from the FDA. This approval would require new testing and compliance inspections; |
|
|
• |
our third-party manufacturers might be unable to timely formulate and manufacture our product or produce the quantity and quality required to meet our clinical and commercial needs, if any; |
|
|
• |
our future third-party manufacturers may not perform as agreed or may not remain in the contract manufacturing business for the time required to supply our clinical trials or to successfully produce, store and distribute our drug or biologic candidates; |
|
|
• |
drug or biologic manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state agencies to ensure strict compliance with cGMPs and other government regulations and corresponding foreign standards, and we do not have direct control over third-party manufacturers’ compliance with these regulations and standards; |
|
|
• |
if any third-party manufacturer makes improvements in the manufacturing process for our products, we may not own or be able to license, or we may have to share, the intellectual property rights to any improvements made by our third-party manufacturers in the manufacturing process for our drug or biologic candidates; |
|
|
• |
while we currently carry insurance in an amount and on terms and conditions that are customary for similarly situated companies and that are satisfactory to our board of directors, we and/or our third-party manufacturers may not have sufficient insurance coverage in the event of any inadvertent destruction of or loss of any drug substance by them, which could result in delays in production and/or our clinical trials and/or result in additional costs to us; and |
|
|
• |
our third-party manufacturers could breach or terminate their agreements with us. |
Each of these risks could delay our clinical trials, the approval, if any, of our drug or biologic candidates, or the commercialization of our drug or biologic candidates or result in higher costs or deprive us of potential product revenue. In addition, we rely on third parties to perform release testing on our drug or biologic candidates prior to delivery to subjects in our clinical trials. If these tests are not appropriately conducted and test data are not reliable, subjects in our clinical trials, or patients treated with our drug or biologic candidates, if any are approved in the future, could be put at risk of serious harm, which could result in product liability suits.
Our employees, independent contractors, principal investigators, CROs, consultants or vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk that our employees, independent contractors, principal investigators, CROs, consultants or vendors may engage in fraudulent or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that violates: FDA regulations, including those laws requiring the reporting of true, complete and accurate information to the FDA; manufacturing standards; federal and state health care fraud and abuse laws and regulations; or laws that require the true, complete and accurate reporting of financial information or data. In addition, sales, marketing and business arrangements in the health care industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Activities subject to these laws also involve the improper use or misrepresentation of information obtained in the course of clinical trials or creating fraudulent data in our preclinical studies or clinical trials, which could result in regulatory sanctions and serious harm to our reputation.
59
It is not always possible to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. Additionally, we are subject to the risk that a person could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal health care programs, contractual damages, reputational harm, diminished potential profits and future earnings, and curtailment of our operations, any of which could adversely affect our business, financial condition, results of operations or prospects.
We have entered into a BMS Collaboration Agreement with Bristol Myers Squibb Company and, pursuant to the terms of that agreement, could become dependent on Bristol Myers Squibb for development, manufacturing, regulatory and commercialization activities with respect to certain of our ETB products directed to multiple targets.
In February 2021, we entered into the BMS Collaboration Agreement, which was amended in December 2021, pursuant to which we agreed to leverage our ETB technology platform to discover and develop novel products directed to multiple targets. Pursuant to the terms of the BMS Collaboration Agreement, we granted Bristol Myers Squibb a series of exclusive options to obtain exclusive licenses under our intellectual property to exploit products containing ETBs directed against certain targets designated by Bristol Myers Squibb. Bristol Myers Squibb may never choose to exercise its option and we cannot predict whether Bristol Myers Squibb will, if ever, exercise its option.
Under the BMS Collaboration Agreement, Bristol Myers Squibb paid us an upfront payment of $70 million. In addition to the upfront payment, we may receive near term and development and regulatory milestone payments of up to an additional $874.5 million. We will also be eligible to receive up to an additional $450 million in payments upon the achievement of certain sales milestones. We will also be entitled to receive, subject to certain reductions, tiered royalties ranging from mid-single digits up to mid-teens as percentages of calendar year net sales, if any, on any licensed product. The milestones that trigger a payment or royalties under the BMS Collaboration Agreement may never be reached and failure to do so could harm our business and financial condition.
We will be responsible for conducting the research activities through the designation, if any, of one or more development candidates. Upon the exercise by Bristol Myers Squibb of its option for a development candidate, Bristol Myers Squibb will be responsible for all development, manufacturing, regulatory and commercialization activities with respect to that development candidate, subject to the terms of the BMS Collaboration Agreement. We cannot control whether Bristol Myers Squibb will devote sufficient attention or resources to this collaboration or will proceed in an expeditious manner. Even if the FDA or other regulatory agencies approve any of the licensed ETB drug or biologic candidates, Bristol Myers Squibb may elect not to proceed with the commercialization of the resulting product in one or more countries.
Unless earlier terminated, the BMS Collaboration Agreement will expire (i) on a country-by-country basis and licensed product-by-licensed product basis on the date of expiration of the royalty payment obligations under the BMS Collaboration Agreement with respect to such licensed product in such country and (ii) in its entirety upon the earlier of (a) the expiration of the royalty payment obligations under the BMS Collaboration Agreement with respect to all licensed products in all countries or (b) upon Bristol Myers Squibb’s decision not to exercise any option on or prior to the applicable option deadlines. Bristol Myers Squibb has the right to terminate the BMS Collaboration Agreement for convenience upon prior written notice to Company. Either party has the right to terminate the BMS Collaboration Agreement (a) for the insolvency of the other party or (b) subject to specified cure periods, in the event of the other party’s uncured material breach. We have the right upon prior written notice to terminate the BMS Collaboration Agreement in the event that Bristol Myers Squibb or any of its affiliates asserts a challenge against our patents. If Bristol Myers Squibb terminates the BMS Collaboration Agreement, it will result in a delay in or could prevent us from further developing or commercializing products directed to these targets and will delay and could prevent us from obtaining revenues for such product. Further, disputes may arise between us and Bristol Myers Squibb, which may delay or cause the termination of this collaboration, result in significant litigation, cause Bristol Myers Squibb to act in a manner that is not in our best interest or cause us to seek another collaborator or proceed with development, commercialization and funding on our own. If we seek a new collaborator but are unable to do so on acceptable terms, or at all, or do not have sufficient funds to conduct the development or commercialization of product directed to these new targets ourselves, we may have to curtail or abandon that development or commercialization, which could harm our business.
60
We depend on third parties and intend to continue to license or collaborate with third parties and may be unable to realize the potential benefits of any collaboration.
Our business strategy, along with our short- and long-term operating results depend in part on our ability to execute on our existing strategic collaboration and to license or partner with new strategic partners. In addition to the BMS Collaboration Agreement, we expect to seek to collaborate with other partners in the future. Even if we are successful at entering into one or more additional collaborations with respect to the development and/or commercialization of one or more drug or biologic candidates, there is no guarantee that any of these collaborations will be successful. We believe collaborations allow us to leverage our resources and technologies and we anticipate deriving some revenues from research and development fees, license fees, milestone payments, and royalties from our collaborative partner. Collaborations may pose a number of risks, including the following:
|
|
• |
collaboration partners often have significant discretion in determining the efforts and resources that they will apply to the collaboration, and may not commit sufficient resources to the development, marketing or commercialization of the product or products that are subject to the collaboration; |
|
|
• |
collaboration partners may not perform their obligations as expected or may breach or terminate their agreements with us or otherwise fail to conduct their collaborative activities successfully and in a timely manner; |
|
|
• |
any such collaboration may significantly limit our share of potential future profits from the associated program, and may require us to relinquish potentially valuable rights to our current drug or biologic candidates, potential products or proprietary technologies or grant licenses on terms that are not favorable to us; |
|
|
• |
collaboration partners may cease to devote resources to the development or commercialization of our drug or biologic candidates if the collaboration partners view our drug or biologic candidates as competitive with their own products or drug or biologic candidates; |
|
|
• |
disagreements with collaboration partners, including disagreements over proprietary rights, contract interpretation or the course of development, might cause delays or termination of the development or commercialization of drug or biologic candidates, and might result in legal proceedings, which would be time consuming, distracting and expensive; |
|
|
• |
collaboration partners may be impacted by changes in their strategic focus or available funding, or business combinations involving them, which could cause them to divert resources away from the collaboration; |
|
|
• |
collaboration partners may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; |
|
|
• |
the collaborations may not result in us achieving revenues sufficient to justify such transactions; |
|
|
• |
by entering into certain collaborations, we may forego opportunities to collaborate with other third parties who do not wish to be associated with our existing third-party strategic partners; and |
|
|
• |
collaborations may be terminated and, if terminated, may result in a need for us to raise additional capital to pursue further development or commercialization of the applicable drug or biologic candidate. |
There can be no assurance that we will be successful at establishing collaborative arrangements on acceptable terms or at all, that collaborative partners will not terminate funding before the completion of projects, that our collaborative arrangements will result in successful product commercialization, or that we will derive any revenues from such arrangements. Potential collaborators may reject collaborations based upon their assessment of our financial, regulatory or intellectual property position and our internal capabilities. Additionally, the negotiation, documentation and implementation of collaborative arrangements are complex and time-consuming. Our discussions with potential collaborators may not lead to new collaborations on favorable terms and may have the potential to provide collaborators with access to our key intellectual property rights.
We enter into various contracts in the normal course of our business in which we indemnify the other party to the contract. In the event we have to perform under these indemnification provisions, it could have a material adverse effect on our business, financial condition and results of operations.
In the normal course of business, we have and expect to continue periodically to enter into academic, commercial, service, collaboration, licensing, supply, consulting and other agreements that contain indemnification provisions. With respect to our academic and other research agreements, we typically indemnify the institution and related parties from losses arising from claims relating to our drug or biologic candidates, processes or services made, used, or performed pursuant to the agreements, and from claims arising from our or our sublicensees’ exercise of rights under the agreement. With respect to our collaboration agreement, we indemnify our collaboration partner from third-party liability claims that could result from the exploitation of our ETB technology by us or any of our affiliates, licensees, agents, contractors, or consultants, a material breach of the collaboration agreement by us or any of our affiliates, licensees, agents, contractors or consultants or any gross negligence or willful misconduct by us or any of our affiliates, licensees, agents, contractors or consultants. With respect to consultants, we often indemnify them from claims arising from the good faith performance of their services.
61
If our obligations under an indemnification provision exceed applicable insurance coverage or if we were denied insurance coverage, our business, financial condition and results of operations could be adversely affected. Similarly, if we are relying on a collaborator to indemnify us and the collaborator is denied insurance coverage or the indemnification obligation exceeds the applicable insurance coverage, and if the collaborator does not have other assets available to indemnify us, our business, financial condition and results of operations could be adversely affected.
Risks Related to Commercialization of Our Drug Candidates
We currently have limited marketing and sales experience. If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our drug or biologic candidates, we may be unable to generate any revenue.
Although some of our employees may have marketed, launched and sold other pharmaceutical products in the past while employed at other companies, we have no experience selling and marketing our drug or biologic candidates, and we currently have no marketing or sales organization. To successfully commercialize any products that may result from our development programs, we will need to find one or more collaboration partners to commercialize our products or invest in and develop these capabilities, either on our own or with others, which would be expensive, difficult and time consuming. Any failure or delay in the timely development of our internal commercialization capabilities could adversely impact the potential for success of our products.
If commercialization collaboration partners do not commit sufficient resources to commercialize our future drugs or biologics, and if we are unable to develop the necessary marketing and sales capabilities on our own, we will be unable to generate sufficient product revenue to sustain or grow our business. We may be competing with companies that currently have extensive and well-funded marketing and sales operations, particularly in the markets our drug or biologic candidates are intended to address. Without appropriate capabilities, whether directly or through third-party collaboration partners, we may be unable to compete successfully against these more established companies.
We may attempt to form additional collaborations in the future with respect to our drug or biologic candidates, but we may not be able to do so, which may cause us to alter our development and commercialization plans.
We may attempt to form strategic collaborations, create joint ventures or enter into licensing arrangements with third parties with respect to our programs in addition to those that we currently have that we believe will complement or augment our existing business. We may face significant competition in seeking appropriate strategic collaboration partners, and the negotiation process to secure appropriate terms is time consuming and complex. We may not be successful in our efforts to establish such a strategic collaboration for any drug or biologic candidates and programs on terms that are acceptable, or at all. This may be because our drug or biologic candidates and programs may be deemed to be at too early of a stage of development for collaborative effort, our research and development pipeline may be viewed as insufficient, the competitive or intellectual property landscape may be viewed as too intense or risky, and/or third parties may not view our drug or biologic candidates and programs as having sufficient potential for commercialization, including the likelihood of an adequate safety and efficacy profile.
Any delays in identifying suitable collaboration partners and entering into agreements to develop and/or commercialize our drug or biologic candidates could delay the development or commercialization of our drug or biologic candidates, which may reduce their competitiveness even if they reach the market. Absent a strategic collaborator, we would need to undertake development and/or commercialization activities at our own expense. If we elect to fund and undertake development and/or commercialization activities on our own, we may need to obtain additional expertise and additional capital, which may not be available to us on acceptable terms or at all. If we are unable to do so, we may not be able to develop our drug or biologic candidates or bring them to market and our business may be materially and adversely affected.
If the market opportunities for our drug or biologic candidates are smaller than we believe they are, we may not meet our revenue expectations and, even if a drug or biologic candidate receives marketing approval, our business may suffer. Because the patient populations in the market for our drug or biologic candidates may be small, we must be able to successfully identify patients and acquire a significant market share to achieve profitability and growth.
Our estimates for the addressable patient population and our estimates for the prices we can charge for our drug or biologic candidates may differ significantly from the actual market addressable by our drug or biologic candidates and are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including the scientific literature, patient foundations or market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these diseases. The number of patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for each of our drug or biologic candidates may be limited or may not be amenable to treatment with our drug or biologic candidates, and new patients may become increasingly difficult to identify or gain access to, which would adversely affect our business, financial condition, results of operations and prospects.
62
We face substantial competition, and our competitors may discover, develop or commercialize drugs faster or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition from large pharmaceutical companies, specialty pharmaceutical companies, biotechnology companies, universities and other research institutions worldwide with respect to MT-6402, MT-8421, MT-0169, and the other drug or biologic candidates that we may seek to develop or commercialize in the future. We are aware that companies including the following have products marketed or in development that could compete directly or indirectly with ETBs: Merck, Bayer, Takeda, AbbVie, Immunogen, Morphosys, Genmab, Bristol Myers Squibb, Novartis, Regeneron, Janssen, Xencor, Amgen, AstraZeneca, Lilly, Merck KGaA, Pfizer, Sanofi, Merrimack Pharmaceuticals, Spectrum Pharmaceuticals, Cogent Biosciences, Karyopharm, ADC Therapeutics, 2seventy bio, Gilead, GlaxoSmithKline, Incyte, TG Therapeutics, and Verastem. Our competitors may succeed in developing, acquiring or licensing technologies or drug or biological products that are more effective or less costly than MT-6402, MT-8421, MT-0169, or any other drug or biologic candidates that we are currently developing or that we may develop, which could render our drug or biologic candidates obsolete and noncompetitive.
Many of our competitors have materially greater name recognition and financial, manufacturing, marketing, research and drug development resources than we do. Additional mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in our competitors. Large pharmaceutical companies in particular have extensive expertise in preclinical and clinical testing and in obtaining regulatory approvals for drugs, including biologics. In addition, academic institutions, government agencies, and other public and private organizations conducting research may seek patent protection with respect to potentially competitive products or technologies. These organizations may also establish exclusive collaborative or licensing relationships with our competitors.
If our competitors obtain marketing approval from the FDA or comparable foreign regulatory authorities for their drug or biologic candidates more rapidly than we do, it could result in our competitors establishing a strong market position before we are able to enter the market. In addition, third-party payors, including governmental and private insurers, also may encourage the use of generic products. For example, if MT-6402, MT-8421, or MT-0169 is ultimately approved, it may be priced at a significant premium over other competitive products. This may make it difficult for, MT-6402, MT-8421, MT-0169, or any other of our future drugs or biologics to compete with these products. Failure of MT-6402, MT-8421, MT-0169 or any other of our drug or biologic candidates to effectively compete against established treatment options or in the future with new products currently in development would harm our business, financial condition, results of operations and prospects.
The commercial success of any of our current or future drug or biologic candidates will depend upon the degree of market acceptance by physicians, patients, third-party payors, and others in the medical community.
Even with the approvals from the FDA and comparable foreign regulatory authorities, the commercial success of our drugs will depend in part on the health care providers, patients and third-party payors accepting our drug or biologic candidates as medically useful, cost-effective and safe. Any product that we bring to the market may not gain market acceptance by physicians, patients or third-party payors. The degree of market acceptance of any of our drug candidates will depend on a number of factors, including but not limited to:
|
|
• |
the efficacy of the product as demonstrated in clinical trials and potential advantages over competing treatments; |
|
|
• |
the prevalence and severity of the disease and any side effects of the product; |
|
|
• |
the clinical indications for which approval is granted, including any limitations or warnings contained in a product’s approved labeling; |
|
|
• |
the convenience and ease of administration of the product; |
|
|
• |
the cost of treatment; |
|
|
• |
the perceptions by the medical community, physicians, and patients, regarding the safety and effectiveness of our products and the willingness of the patients and physicians to accept these therapies; |
|
|
• |
the perceived ratio of risk and benefit of these therapies by physicians and the willingness of physicians to recommend these therapies to patients based on such risks and benefits; |
|
|
• |
the marketing, sales, supply and distribution support for the product; |
|
|
• |
the publicity concerning our drugs or biologics or competing products and treatments; and |
|
|
• |
the pricing and availability of third-party insurance coverage and reimbursement. |
Even if a product displays a favorable efficacy and safety profile upon approval, market acceptance of the product remains uncertain. Efforts to educate the medical community and third-party payors on the benefits of the drugs may require significant investment and resources and may never be successful. If our drugs or biologics fail to achieve an adequate level of acceptance by physicians, patients, third-party payors and other health care providers, we will not be able to generate sufficient revenue to become or remain profitable.
63
Our ability to negotiate, secure and maintain third-party coverage and reimbursement for our drug or biologic candidates may be affected by political, economic and regulatory developments in the United States, the European Union and other jurisdictions. Governments continue to impose cost containment measures, and third-party payors are increasingly challenging prices charged for medicines and examining their cost effectiveness, in addition to their safety and efficacy. These and other similar developments could significantly limit the degree of market acceptance of any drug or biologic candidate of ours that receives marketing approval in the future.
We may not be successful in any efforts to identify, license, discover, develop or commercialize additional drug or biologic candidates.
Although a substantial amount of our effort has focused on the continued clinical testing, potential approval and commercialization of our existing drug or biologic candidates, the success of our business is also expected to depend in part upon our ability to identify, license, discover, develop or commercialize additional drug or biologic candidates. Research programs to identify new drug or biologic candidates require substantial technical, financial and human resources. We may focus our efforts and resources on potential programs or drug or biologic candidates that ultimately prove to be unsuccessful. Our research programs or licensing efforts may fail to yield additional drug or biologic candidates for clinical development and commercialization for a number of reasons, including but not limited to the following:
|
|
• |
our research or business development methodology or search criteria and process may be unsuccessful in identifying potential drug or biologic candidates; |
|
|
• |
we may not be able or willing to assemble sufficient resources to acquire or discover additional drug or biologic candidates; |
|
|
• |
our drug or biologic candidates may not succeed in preclinical or clinical testing; |
|
|
• |
our drug or biologic candidates may be shown to have harmful side effects or may have other characteristics that may make them unmarketable or unlikely to receive marketing approval; |
|
|
• |
competitors may develop alternatives that render our drug or biologic candidates obsolete or less attractive; |
|
|
• |
drug or biologic candidates we develop may be covered by third-parties’ patents or other exclusive rights; |
|
|
• |
the market for a drug or biologic candidate may change during our program so that such a drug or biologic candidate may become unreasonable or infeasible to continue to develop; |
|
|
• |
a drug or biologic candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and |
|
|
• |
a drug or biologic candidate may not be accepted as safe and effective by patients, the medical community or third-party payors. |
If any of these events occur, we may be forced to abandon our development efforts for a program or programs, or we may not be able to identify, license, discover, develop or commercialize additional drug or biologic candidates, which would have a material adverse effect on our business, financial condition or results of operations and could potentially cause us to cease operations.
Failure to obtain or maintain adequate reimbursement or insurance coverage for drugs, if any, could limit our ability to market those drugs and decrease our ability to generate revenue.
The pricing, coverage, and reimbursement of our approved drugs, if any, must be sufficient to support our commercial efforts and other development programs, and the availability and adequacy of coverage and reimbursement by third-party payors, including governmental and private insurers, are essential for most patients to be able to afford medical treatments. Sales of our approved drugs, if any, will depend substantially, both domestically and abroad, on the extent to which the costs of our approved drugs, if any, will be paid for or reimbursed by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or government payors and private payors. If coverage and reimbursement are not available, or are available only in limited amounts, we may have to subsidize or provide drugs for free or we may not be able to successfully commercialize our drugs.
In addition, there is significant uncertainty related to the insurance coverage and reimbursement for newly approved drugs. In the United States, the principal decisions about coverage and reimbursement for new drugs are typically made by the Centers for Medicare and Medicaid Services (“CMS”) an agency within the United States Department of Health and Human Services, as CMS decides whether and to what extent a new drug will be covered and reimbursed under Medicare. Private payors tend to follow the coverage reimbursement policies established by CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to reimbursement for novel drug or biologic candidates such as ours and what reimbursement codes our drug or biologic candidates may receive if approved. Moreover, as noted above under “Healthcare legislative reform measures may have a material adverse effect on our business, financial condition or results of operations” Congress recently enacted and President Biden signed into law new authorities for CMS to negotiate drug prices annually for certain prescription drugs and biological products, subject to statutory criteria and a future selection process that is in the process of being developed by CMS. It is unclear how these forthcoming
64
changes in the way that CMS does business with certain members of the biopharmaceutical industry may impact coverage or reimbursement decisions across the industry as a whole.
Outside the United States, international operations are generally subject to extensive governmental price controls and other price-restrictive regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada and other countries has and will continue to put pressure on the pricing and usage of drugs. In many countries, the prices of drugs are subject to varying price control mechanisms as part of national health systems. Price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our drugs, if any. Accordingly, in markets outside the United States, the potential revenue may be insufficient to generate commercially reasonable revenue and profits.
Moreover, increasing efforts by governmental and private payors in the United States and abroad to limit or reduce healthcare costs may result in restrictions on coverage and the level of reimbursement for new drugs and, as a result, they may not cover or provide adequate payment for our drugs, if any. We expect to experience pricing pressures in connection with drugs due to the increasing trend toward managed healthcare, including the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, and prescription drugs or biologics in particular, has and is expected to continue to increase in the future. As a result, profitability of our drugs, if any, may be more difficult to achieve even if any of them receive regulatory approval.
Risks Related to Ownership of Our Common Stock
The market price of our common stock is expected to be volatile, and the market price of the common stock may drop.
The market price of our common stock could be subject to significant fluctuations. Market prices for securities of early-stage pharmaceutical, biotechnology and other life sciences companies have historically been particularly volatile. Some of the factors that may cause the market price of our common stock to fluctuate include:
|
|
• |
our ability to obtain regulatory approvals for MT-6402, MT-8421, MT-0169, or other drug or biologic candidates, and delays or failures to obtain such approvals; |
|
|
• |
adverse results, clinical holds, or delays in the clinical trials of our drug or biologic candidates or any future clinical trials we may conduct, or changes in the development status of our drug or biologic candidates; |
|
|
• |
failure of any of our drug or biologic candidates, if approved, to achieve commercial success; |
|
|
• |
failure to maintain our existing third-party collaboration, license and supply agreements; |
|
|
• |
failure by us or our licensors to prosecute, maintain, or enforce our intellectual property rights; |
|
|
• |
changes in laws or regulations applicable to our drug or biologic candidates; |
|
|
• |
any inability to obtain adequate supply of our drug or biologic candidates or the inability to do so at acceptable prices; |
|
|
• |
adverse regulatory authority decisions; |
|
|
• |
introduction of new products, services or technologies by our competitors; |
|
|
• |
failure to meet or exceed financial and development projections we may provide to the public; |
|
|
• |
failure to meet or exceed the financial and development projections of the investment community; |
|
|
• |
the perception of the pharmaceutical industry by the public, legislatures, regulators and the investment community; |
|
|
• |
announcements of significant acquisitions, strategic collaborations, strategic alternatives, joint ventures or capital commitments by us or our competitors; |
|
|
• |
disputes or other developments relating to proprietary rights, including patents, litigation matters, and our ability to obtain patent protection for our technologies; |
|
|
• |
additions or departures of key personnel; |
|
|
• |
significant lawsuits, including patent or stockholder litigation; |
|
|
• |
failure by securities or industry analysts to publish research or reports about our business, or issuance of any adverse or misleading opinions by such analysts regarding our business or stock; |
|
|
• |
changes in the market valuations of similar companies; |
|
|
• |
general market or macroeconomic conditions, such as inflation; |
|
|
• |
sales of our common stock by us or our stockholders in the future; |
65
|
|
• |
the trading volume of our common stock; |
|
|
• |
the issuance of additional shares of our preferred stock or common stock, or the perception that such issuances may occur, including through our “at-the-market” offering program or any sales of our preferred stock or common stock by our stockholders in the future; |
|
|
• |
announcements by commercial partners or competitors of new commercial products, clinical progress or the lack thereof, significant contracts, commercial relationships or capital commitments; |
|
|
• |
adverse publicity relating to ETB drugs generally, including with respect to other drugs and potential drugs in such markets; |
|
|
• |
the introduction of technological innovations or new therapies that compete with our potential drugs; |
|
|
• |
changes in the structure of health care payment systems; |
|
|
• |
disruptions in the financial markets in general and more recently due to the COVID-19 pandemic; |
|
|
• |
the impact of political instability and military conflict, such as the conflict in Ukraine, which has resulted in instability in the global financial markets and export controls; and |
|
|
• |
period-to-period fluctuations in our financial results. |
Moreover, the stock markets in general have experienced substantial volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations may also adversely affect the trading price of our common stock.
Future sales of a substantial number of shares of our common stock in the public market, or the perception that such sales could occur, could cause our stock price to fall.
If our existing stockholders sell, or indicate an intention to sell, substantial amounts of our common stock in the public market, the trading price of our common stock could decline. As of March 31, 2023, a total of 56,351,647 shares of our common stock were outstanding. Any sales of those shares or any perception in the market that such sales may occur could cause the trading price of our common stock to decline.
In addition, shares of our common stock that are either subject to outstanding options or reserved for future issuance under our equity incentive plan will be eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules. If these additional shares of common stock are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common stock could decline.
We may become involved in securities litigation that could divert management’s attention and harm the company’s business, and insurance coverage may not be sufficient to cover all costs and damages.
We may be exposed to securities litigation even if no wrongdoing occurred. Litigation is usually expensive and diverts management’s attention and resources, which could adversely affect our business and cash resources. We may become involved in such litigation, and our stock price may fluctuate for many reasons, including as a result of public announcements regarding the progress of our development efforts or the development efforts of current or future collaboration partners or competitors, the addition or departure of our key personnel, the announcement of the strategic restructuring, variations in our quarterly operating results and changes in market valuations of biopharmaceutical and biotechnology companies.
This risk is especially relevant to us because biopharmaceutical and biotechnology companies have experienced significant stock price volatility in recent years. When the market price of a stock has been volatile, as our stock price may be, holders of that stock have occasionally brought securities class action litigation against the company that issued the stock. If any of our stockholders were to bring a lawsuit of this type against us, even if the lawsuit is without merit, it could result in substantial costs for defending the lawsuit and diversion of the time, attention and resources of our board of directors and management, which could significantly harm our profitability and reputation.
Our amended and restated certificate of incorporation, amended and restated bylaws and Delaware law contain provisions that could discourage another company from acquiring us and may prevent attempts by our stockholders to replace or remove our current management.
Provisions of Delaware law, where we are incorporated, our amended and restated certificate of incorporation and our amended and restated bylaws may discourage, delay or prevent a merger or acquisition that our stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace or remove our board of directors. These provisions include:
|
|
• |
authorizing our board of directors to issue “blank check” preferred stock without any need for approval by stockholders; |
|
|
• |
providing for a classified board of directors with staggered three-year terms; |
66
|
|
• |
requiring supermajority stockholder votes to effect certain amendments to our amended and restated certificate of incorporation and amended and restated bylaws; |
|
|
• |
eliminating the ability of stockholders to call special meetings of stockholders; |
|
|
• |
prohibiting stockholder action by written consent; and |
|
|
• |
establishing advance notice requirements for nominations for election to our board of directors or for proposing matters that can be acted on by stockholders at stockholder meetings. |
We can issue and have issued shares of preferred stock, which may adversely affect the rights of holders of our common stock.
Our amended and restated certificate of incorporation authorizes us to issue up to 2,000,000 shares of preferred stock with designations, rights, and preferences determined from time-to-time by our Board of Directors. Accordingly, our Board of Directors is empowered, without stockholder approval, to issue preferred stock with dividend, liquidation, conversion, voting or other rights superior to those of holders of our common stock. For example, an issuance of shares of preferred stock could:
|
|
• |
adversely affect the voting power of the holders of our common stock; |
|
|
• |
make it more difficult for a third party to gain control of us; |
|
|
• |
discourage bids for our common stock at a premium; |
|
|
• |
limit or eliminate any payments that the holders of our common stock could expect to receive upon our liquidation; or |
|
|
• |
otherwise adversely affect the market price or our common stock. |
Claims for indemnification by our directors and officers may reduce our available funds to satisfy successful third-party claims against us and may reduce the amount of money available to us.
Our amended and restated certificate of incorporation and amended and restated bylaws provide that we will indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law.
In addition, as permitted by Section 145 of the Delaware General Corporation Law, or the DGCL, our amended and restated bylaws and our indemnification agreements that we have entered into with our directors and executive officers provide that:
|
|
• |
We will indemnify our directors and executive officers for serving us in those capacities or for serving other related business enterprises at our request, to the fullest extent permitted by Delaware law. Delaware law provides that a corporation may indemnify such person if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the registrant and, with respect to any criminal proceeding, had no reasonable cause to believe such person’s conduct was unlawful. |
|
|
• |
We may, in our discretion, indemnify employees and agents in those circumstances where indemnification is permitted by applicable law. |
|
|
• |
We are required to advance expenses, as incurred, to our directors and officers in connection with defending a proceeding, except that such directors or officers shall undertake to repay such advances if it is ultimately determined that such person is not entitled to indemnification. |
|
|
• |
The rights conferred in our amended and restated bylaws are not exclusive, and we are authorized to enter into indemnification agreements with our directors, officers, employees and agents and to obtain insurance to indemnify such persons. |
|
|
• |
We may not retroactively amend our amended and restated bylaw provisions to reduce our indemnification obligations to directors, officers, employees and agents. |
We have never paid dividends on our common stock, and we do not anticipate paying any cash dividends in the foreseeable future.
We have never declared or paid cash dividends on our common stock. We do not anticipate paying any cash dividends on our common stock in the foreseeable future. We currently intend to retain all available funds and any future earnings to fund the development and growth of our business. As a result, capital appreciation, if any, of our common stock will be our stockholders’ sole source of gain for the foreseeable future.
67
We incur, and will continue to incur, costs and expect significantly increased costs as a result of operating as a public company, and our management is now required to devote substantial time to new compliance initiatives.
As a public company listed on The Nasdaq Capital Market, and particularly after we cease to be a “smaller reporting company,” we are incurring and will continue to incur significant legal, accounting and other expenses that we did not incur as a private company or as a public company prior to the loss of such specified statuses. We are subject to the reporting requirements of the Exchange Act, as well as various requirements imposed by the Sarbanes-Oxley Act, rules subsequently adopted by the SEC and Nasdaq to implement provisions of the Sarbanes-Oxley Act, and the Dodd-Frank Wall Street Reform and Consumer Protection Act. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate. The listing requirements of The Nasdaq Capital Market require that we satisfy certain corporate governance requirements relating to director independence, distributing annual and interim reports, stockholder meetings, approvals and voting, soliciting proxies, conflicts of interest and a code of conduct, each of which requires additional attention and effort of management and our board of directors and additional costs.
We expect the rules and regulations applicable to public companies to substantially increase our legal and financial compliance costs and to make some activities more time-consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain the same or similar coverage. We also expect that we will need to hire additional accounting, finance and other personnel in connection with our efforts to comply with the requirements of being a public company, and our management and other personnel will need to devote a substantial amount of time towards maintaining compliance with these requirements. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors and committees thereof or as executive officers.
Our executive officers, directors and principal stockholders have the ability to significantly influence all matters submitted to our stockholders for approval.
As of March 31, 2023, our directors, executive officers, and stockholders beneficially owning 5% or more of our shares or that may be affiliated with our board members, beneficially owned, in the aggregate, approximately 52% of our outstanding shares of common stock. As a result, if these stockholders were to choose to act together, they would be able to significantly influence almost all matters submitted to our stockholders for approval, as well as our management and affairs. For example, these persons, if they choose to act together, would significantly influence the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets.
Our amended and restated bylaws provide that the Court of Chancery of the State of Delaware is the exclusive forum for specified disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or other employees.
Our amended and restated bylaws provide, to the fullest extent permitted by law, that the Court of Chancery of the State of Delaware will be the exclusive forum for: (1) any derivative action or proceeding brought on our behalf; (2) any action asserting a breach of fiduciary duty; (3) any action asserting a claim against us arising pursuant to the Delaware General Corporation Law (the “DGCL”), our amended and restated certificate of incorporation, or our amended and restated bylaws; or (4) any action asserting a claim against us that is governed by the internal affairs doctrine. This exclusive forum provision does not apply to suits brought to enforce a duty or liability created by the Exchange Act. It could apply, however, to a suit that falls within one or more of the categories enumerated in the exclusive forum provision and asserts claims under the Securities Act, in as much as Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rule and regulations thereunder. There is uncertainty as to whether a court would enforce such provision with respect to claims under the Securities Act, and our stockholders will not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder. This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or any of our directors, officers, or other employees, which may discourage lawsuits with respect to such claims. Alternatively, if a court were to find the choice of forum provisions contained in our amended and restated bylaws to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations and financial condition.
68
If securities or industry analysts do not publish, or cease publishing, research or reports, or publish unfavorable research or reports, about us, our business or our market, or if they change their recommendations regarding our stock adversely, our stock price and trading volume could decline.
The trading market for our common stock will be influenced, in part, by the research and reports that industry or financial research analysts publish about us and our business. We do not have any control over these analysts. If only a few securities or industry analysts commence coverage of our company, the trading price for our stock would likely be negatively affected and there can be no assurance that analysts will provide favorable coverage. If securities or industry analysts who initiate coverage downgrade our stock or publish inaccurate or unfavorable research about our business or our market, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and any trading volume to decline.
Having availed ourselves of scaled disclosure available to smaller reporting companies, we cannot be certain if such reduced disclosure will make our common stock less attractive to investors.
Under Section 12b-2 of the Exchange Act, a "smaller reporting company" is a company that is not an investment company, an asset backed issuer, or a majority-owned subsidiary of a parent company. Effective September 10, 2018, the definition of a “smaller reporting company” was amended to include companies with a public float of less than $250 million as of the last business day of its most recently completed second fiscal quarter or, if such public float is less than $700 million, had annual revenues of less than $100 million during the most recently completed fiscal year. Smaller reporting companies are permitted to provide simplified executive compensation disclosure in their filings; they are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal controls over financial reporting; and they have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. As calculated as of June 30, 2022, we qualified as a smaller reporting company. For as long as we continue to be a smaller reporting company, we expect that we will take advantage of the reduced disclosure obligations available to us as a result of those respective classifications. Decreased disclosure in our SEC filings as a result of our having availed ourselves of scaled disclosure may make it harder for investors to analyze our results of operations and financial prospects.
Risks Related to Our Business Operations
Our future success depends in part on our ability to retain our Chief Executive Officer and Chief Scientific Officer and to retain and motivate other qualified personnel.
We are highly dependent on Eric E. Poma, Ph.D., our Chief Executive Officer and Chief Scientific Officer, the loss of whose services may adversely impact the achievement of our objectives. Dr. Poma could leave our employment at any time, as he is an “at will” employee. Recruiting and retaining other qualified employees, consultants and advisors for our business, including scientific and technical personnel, will also be crucial to our success. Our recently announced strategic prioritization and restructuring may result in the loss of personnel with deep institutional or technical knowledge. Further, the transition could potentially disrupt our operations and relationships with employees, suppliers and partners and due to added costs, operational inefficiencies, decreased employee morale and productivity and increased turnover. Furthermore, these personnel changes may increase our dependency on the other members of our leadership team and other employees that remain with us, who are not contractually obligated to remain employed with us and may leave at any time. Any such departure could be particularly disruptive and, to the extent we experience additional turnover, competition for top talent is high such that it may take some time to find a candidate that meets our requirements. Our competitors may seek to use these transitions and the related potential disruptions to gain a competitive advantage over us. There is currently a shortage of highly qualified personnel in our industry, which is likely to continue. Additionally, this shortage of highly qualified personnel is particularly acute in the area where we are located. As a result, competition for personnel is intense and the turnover rate can be high. We may not be able to attract and retain personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for individuals with similar skill sets.
In addition, failure to succeed in development and commercialization of our drug or biologic candidates may make it more challenging to recruit and retain qualified personnel. The inability to recruit and retain qualified personnel, or the loss of the services of Dr. Poma may impede the progress of our research, development and commercialization objectives and would negatively impact our ability to succeed in our product development strategy.
We rely significantly on information technology and any failure, inadequacy, interruption or security lapse of that technology or loss of data, including any cyber security incidents, could compromise sensitive information related to our business, prevent us from accessing critical information or expose us to liability which could harm our ability to operate our business effectively and adversely affect our business and reputation.
Our ability to execute our business plan and maintain operations depends on the continued and uninterrupted performance of our information technology (“IT”) systems, some of which are in our control and some of which are in the control of third parties. In the ordinary course of our business, we collect and store sensitive data, including personally identifiable information about our employees, intellectual property, and proprietary business information (“Confidential Information”). We manage and maintain our applications and data utilizing on-site systems and we also have outsourced elements of our operations to third parties, and as a result we manage a
69
number of third-party vendors who may or could have access to our Confidential Information. These applications and data encompass a wide variety of business-critical information including research and development information and business and financial information.
The secure processing, storage, maintenance and transmission of this critical information is vital to our operations and business strategy. Despite the implementation of security measures, including the implementation of a Company cybersecurity program, which includes network penetration testing, detecting and addressing threats and cybersecurity training for employees, our IT systems are vulnerable to risks and damages from a variety of sources, including telecommunications or network failures, cyber-attacks, computer viruses, ransomware attacks, phishing schemes, breaches, unauthorized access, interruptions due to employee error or malfeasance or other disruptions, or damage from natural disasters, terrorism, war and telecommunication and electrical failures, or other attempts to harm or access our systems. Moreover, despite network security and back-up measures, some of our servers and those of our business partners are potentially vulnerable to physical or electronic break-ins, including cyber-attacks, computer viruses and similar disruptive problems. These events could lead to the unauthorized access, disclosure and use of Confidential Information. Breaches resulting in the compromise, disruption, degradation, manipulation, loss, theft, destruction, or unauthorized disclosure or use of Confidential Information, or the unauthorized access to, disruption of, or interference with any future products and services, can occur in a variety of ways, including but not limited to, negligent or wrongful conduct by employees or others with permitted access to our IT systems and information, or wrongful conduct by hackers, competitors, or certain governments. Our third-party vendors and business partners face similar risks.
Cyber-attacks come in many forms, including the deployment of harmful malware or ransomware, exploitation of vulnerabilities, phishing and other use of social engineering, and other means to compromise the confidentiality, integrity, and availability of our IT systems and Confidential Information. The techniques used by criminal elements to attack computer systems are sophisticated, change frequently and may originate from less regulated or remote areas of the world. As a result, even with appropriate monitoring controls, we may not be able to address these techniques proactively or implement adequate preventative measures. There can be no assurance that we will promptly detect or intercept any such disruption or security breach, if at all. If our computer systems are compromised, we could be subject to fines, damages, reputational harm, litigation and enforcement actions, and we could lose trade secrets, the occurrence of which could harm our business, in addition to possibly requiring substantial expenditures of resources to remedy. For example, any such event that leads to unauthorized access, use or disclosure of personal information, including personal information regarding our patients, to the extent we have such information, or employees, could harm our reputation, require us to comply with federal and/or state breach notification laws and foreign law equivalents, and otherwise subject us to liability under laws and regulations that protect the privacy and security of personal information. In addition, the loss of data from clinical trials for our drug or biologic candidates could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce data and a cybersecurity breach could adversely affect our reputation and could result in other negative consequences, including disruption of our internal operations, increased cyber security protection costs, lost revenues or litigation. Despite precautionary measures to prevent unanticipated problems that could affect our IT systems, sustained or repeated system failures that interrupt our ability to generate and maintain data could adversely affect our ability to operate our business.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS.
None.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. OTHER INFORMATION
Departure of David Hirsch, M.D., Ph.D. from the Board of Directors
On May 13, 2023, David Hirsch, M.D., Ph.D. notified the Company that he would be resigning from the Board of Directors of the Company effective on May 14, 2023. Prior to his resignation, Dr. Hirsch served as a Class III director of the Company and as a member of the Audit Committee and the Nominating and Corporate Governance Committee of the Board of Directors. The Company expressed gratitude to Dr. Hirsch for his contributions to the Board of Directors and the Company. Dr. Hirsch’s resignation did not involve a disagreement with the Company on any matter relating to the Company’s operations, policies or practices.
70
ITEM 6. EXHIBITS
EXHIBIT INDEX
The exhibits listed on the accompanying index to exhibits are filed or incorporated by reference (as stated therein) as part of this Quarterly Report on Form 10-Q.
|
Exhibit Number |
|
Description |
|
|
|
|
|
|
|
|
|
31.1* |
|
|
|
|
|
|
|
31.2* |
|
|
|
|
|
|
|
32.1** |
|
|
|
|
|
|
|
101.INS |
|
Inline XBRL Instance Document. |
|
|
|
|
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document. |
|
|
|
|
|
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
|
|
|
|
|
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document. |
|
|
|
|
|
101.LAB |
|
Inline XBRL Taxonomy Extension Labels Linkbase Document. |
|
|
|
|
|
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
* Filed herewith.
|
** |
Furnished herewith. This certification is not deemed filed for purposes of Section 18 of the Exchange Act, or otherwise subject to the liability of that section, and is not deemed to be incorporated by reference into any filing under the Securities the Exchange Act. |
71
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
Molecular Templates, Inc. |
|
|
|
|
|
Date: May 15, 2023 |
|
/s/ Eric E. Poma |
|
|
|
Eric E. Poma, Ph.D. |
|
|
|
Chief Executive Officer and Chief Scientific Officer |
|
|
|
|
|
Date: May 15, 2023 |
|
/s/ Jason S. Kim |
|
|
|
Jason S. Kim |
|
|
|
Interim Chief Financial Officer |
72