
EXHIBIT 99.2

R&D Day Presentation 2022 The Society for Immunotherapy of Cancer’s (SITC) 37th Annual Meeting

Forward looking statements 2 Except for statements of historical fact, the statements in this presentation are forward - looking statements, including, but not limited to, statements regarding the future development of our proprietary Engineered Toxin Body (ETB) technology; statements relating to the development of MT - 6402, MT - 5111, MT - 0169, and MT - 8421 and our next generation ETBs and preclinical pipeline; statements regarding the safety or potential efficacy of our drug or biologic candidates, including the anticipated benefits of our next - generation ETBs; our belief that our proprietary ETB technology provides for a differentiated mechanism of action that may address some of the limitations associated with currently available cancer therapeutics; statements regarding expected demand and opportunities for certain targets; expected program milestones; the timing, progress and results of pre - clinical studies and clinical trials for our drug or biologic candidates or any future candidates; the timing or likelihood of regulatory filings, including expected timing for submission and approval of various IND applications; the expected participated and presentation at upcoming conferences; our expected receipt of clinical data; the expected timing for providing updates on our pipeline, including MT - 6402, MT - 5111, MT - 0169 and MT - 8421, and our earlier stage pipeline of ETBs; and statements relating to the outcome of our collaborations as they relate to our ETB platform. These statements constitute "forward - looking statements" within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act and are usually identified by the use of words such as "anticipates," "believes," "estimates," "expects," "intends," "may," "plans," "projects," "seeks," "should," "will," and variations of such words or similar expressions. These forward - looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward - looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward - looking statements and will be affected by a variety of risks and factors that are beyond our control. These statements involve risks and uncertainties that can cause actual results to differ materially from those in such forward - looking statements. Important factors that may cause actual results to differ materially from the results discussed in the forward - looking statements include risks and uncertainties, including (1) our failure to secure and maintain relationships with collaborators; (2) risks relating to clinical trials and other uncertainties of drug or biologic candidate development; (3) risks relating to the commercialization, if any, of our proposed drug or biologic candidates (such as marketing, regulatory, product liability, supply, competition, and other risks); (4) dependence on the efforts of third parties including our strategic partners; (5) dependence on intellectual property; and (6) risks from global pandemics including COVID - 19. Further information regarding these and other risks is included under the heading "Risk Factors" in our filings with the Securities and Exchange Commission available from the SEC's website (www.sec.gov). Existing and prospective investors are cautioned not to place undue reliance on these forward - looking statements, which speak only as of the date hereof. These forward looking statements reflect management’s current views and we do not undertake to update any of these forward - looking statements to reflect a change in events or circumstances that occur after the date of this presentation except as required by law.
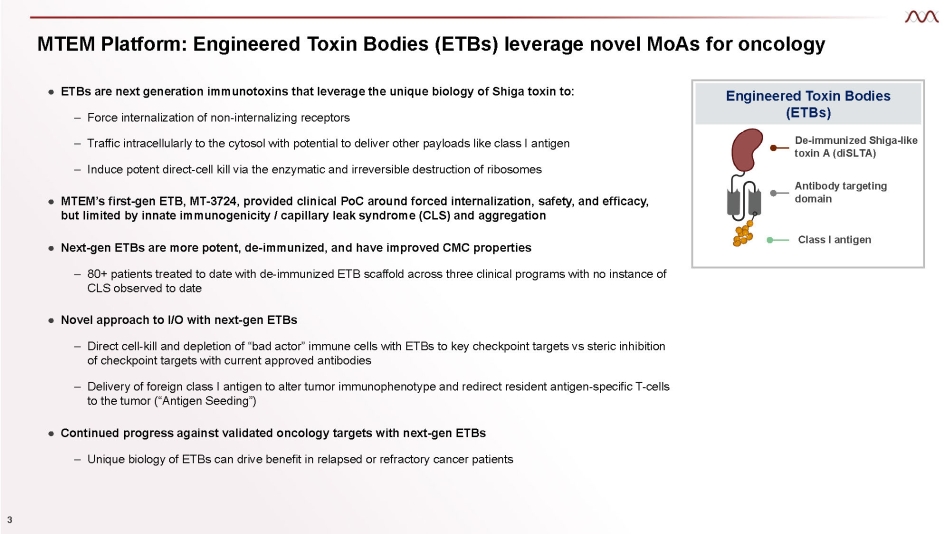
MTEM Platform: Engineered Toxin Bodies (ETBs) leverage novel MoAs for oncology Engineered Toxin Bodies (ETBs) De - immunized Shiga - like toxin A (diSLTA) Antibody targeting domain Class I antigen ● ETBs are next generation immunotoxins that leverage the unique biology of Shiga toxin to: 3 – Force internalization of non - internalizing receptors – Traffic intracellularly to the cytosol with potential to deliver other payloads like class I antigen – Induce potent direct - cell kill via the enzymatic and irreversible destruction of ribosomes ● MTEM’s first - gen ETB, MT - 3724, provided clinical PoC around forced internalization, safety, and efficacy, but limited by innate immunogenicity / capillary leak syndrome (CLS) and aggregation ● Next - gen ETBs are more potent, de - immunized, and have improved CMC properties – 80+ patients treated to date with de - immunized ETB scaffold across three clinical programs with no instance of CLS observed to date ● Novel approach to I/O with next - gen ETBs – Direct cell - kill and depletion of “bad actor” immune cells with ETBs to key checkpoint targets vs steric inhibition of checkpoint targets with current approved antibodies – Delivery of foreign class I antigen to alter tumor immunophenotype and redirect resident antigen - specific T - cells to the tumor (“Antigen Seeding”) ● Continued progress against validated oncology targets with next - gen ETBs – Unique biology of ETBs can drive benefit in relapsed or refractory cancer patients
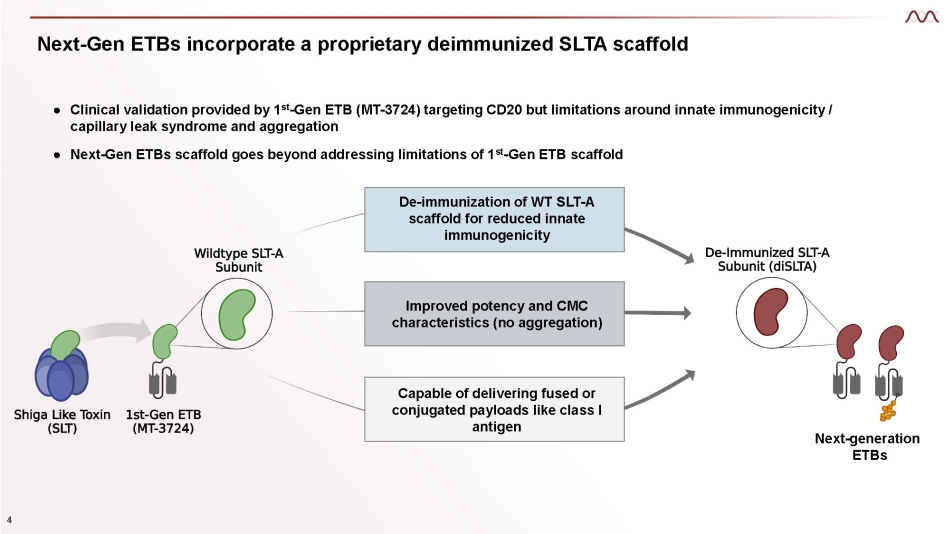
De - immunization of WT SLT - A scaffold for reduced innate immunogenicity Next - Gen ETBs incorporate a proprietary deimmunized SLTA scaffold Improved potency and CMC characteristics (no aggregation) Capable of delivering fused or conjugated payloads like class I antigen ● Clinical validation provided by 1 st - Gen ETB (MT - 3724) targeting CD20 but limitations around innate immunogenicity / capillary leak syndrome and aggregation ● Next - Gen ETBs scaffold goes beyond addressing limitations of 1 st - Gen ETB scaffold Next - generation 4 ETBs
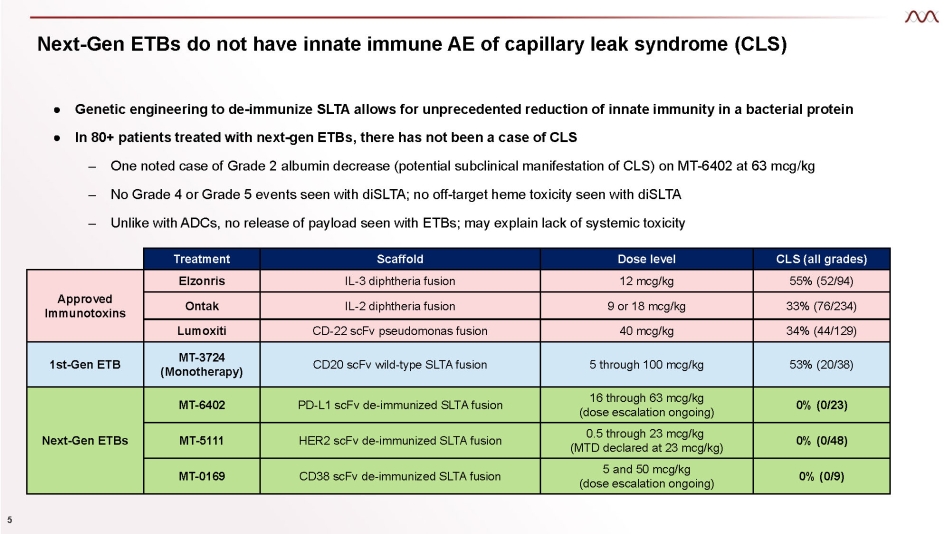
● Genetic engineering to de - immunize SLTA allows for unprecedented reduction of innate immunity in a bacterial protein ● In 80+ patients treated with next - gen ETBs, there has not been a case of CLS – One noted case of Grade 2 albumin decrease (potential subclinical manifestation of CLS) on MT - 6402 at 63 mcg/kg – No Grade 4 or Grade 5 events seen with diSLTA; no off - target heme toxicity seen with diSLTA – Unlike with ADCs, no release of payload seen with ETBs; may explain lack of systemic toxicity 5 Next - Gen ETBs do not have innate immune AE of capillary leak syndrome (CLS) Treatment Scaffold Dose level CLS (all grades) Approved Immunotoxins Elzonris IL - 3 diphtheria fusion 12 mcg/kg 55% (52/94) Ontak IL - 2 diphtheria fusion 9 or 18 mcg/kg 33% (76/234) Lumoxiti CD - 22 scFv pseudomonas fusion 40 mcg/kg 34% (44/129) 1st - Gen ETB MT - 3724 (Monotherapy) CD20 scFv wild - type SLTA fusion 5 through 100 mcg/kg 53% (20/38) Next - Gen ETBs MT - 6402 PD - L1 scFv de - immunized SLTA fusion 16 through 63 mcg/kg (dose escalation ongoing) 0% (0/23) MT - 5111 HER2 scFv de - immunized SLTA fusion 0.5 through 23 mcg/kg (MTD declared at 23 mcg/kg) 0% (0/48) MT - 0169 CD38 scFv de - immunized SLTA fusion 5 and 50 mcg/kg (dose escalation ongoing) 0% (0/9)
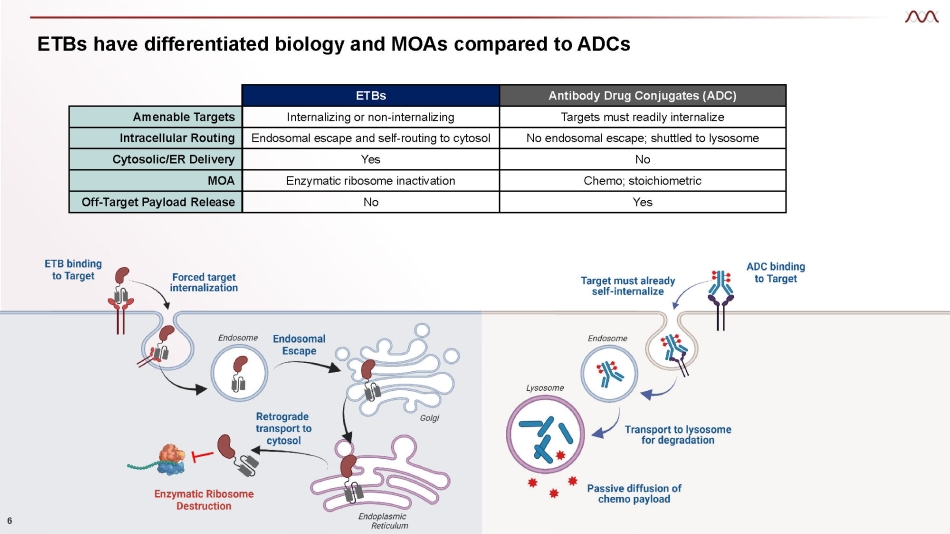
ETBs have differentiated biology and MOAs compared to ADCs 6 ETBs Antibody Drug Conjugates (ADC) Amenable Targets Internalizing or non - internalizing Targets must readily internalize Intracellular Routing Endosomal escape and self - routing to cytosol No endosomal escape; shuttled to lysosome Cytosolic/ER Delivery Yes No MOA Enzymatic ribosome inactivation Chemo; stoichiometric Off - Target Payload Release No Yes
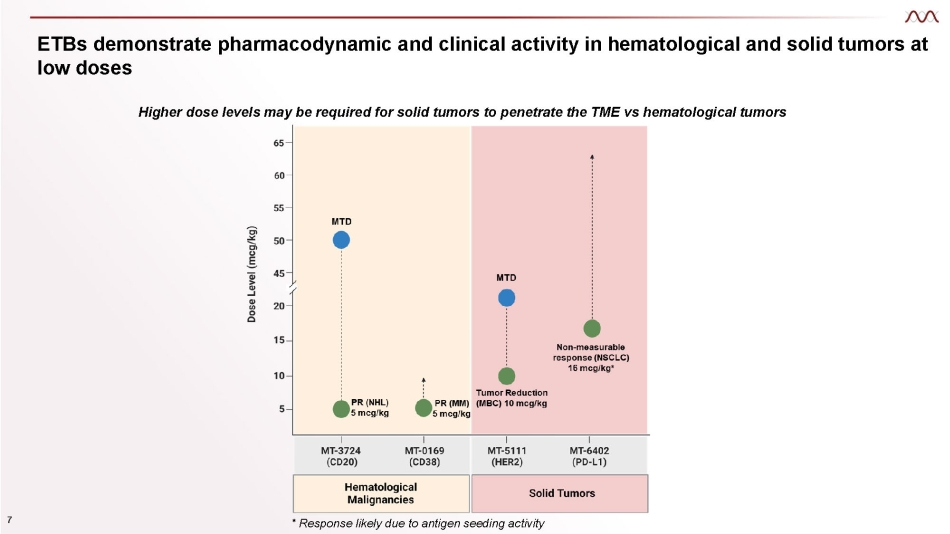
ETBs demonstrate pharmacodynamic and clinical activity in hematological and solid tumors at low doses Higher dose levels may be required for solid tumors to penetrate the TME vs hematological tumors 7 * Response likely due to antigen seeding activity

Novel approach to I - O targets Dismantling the TME and altering tumor immunophenotype
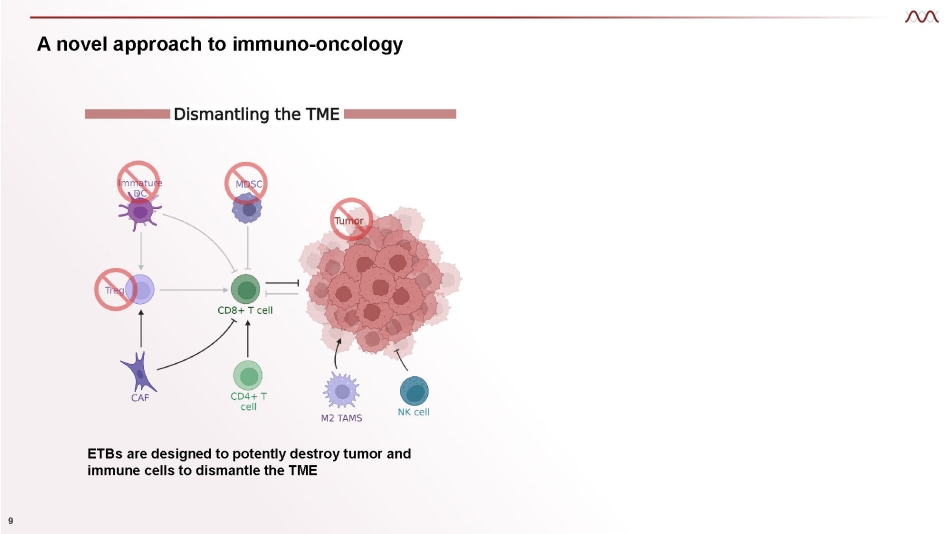
A novel approach to immuno - oncology ETBs are designed to potently destroy tumor and immune cells to dismantle the TME 9
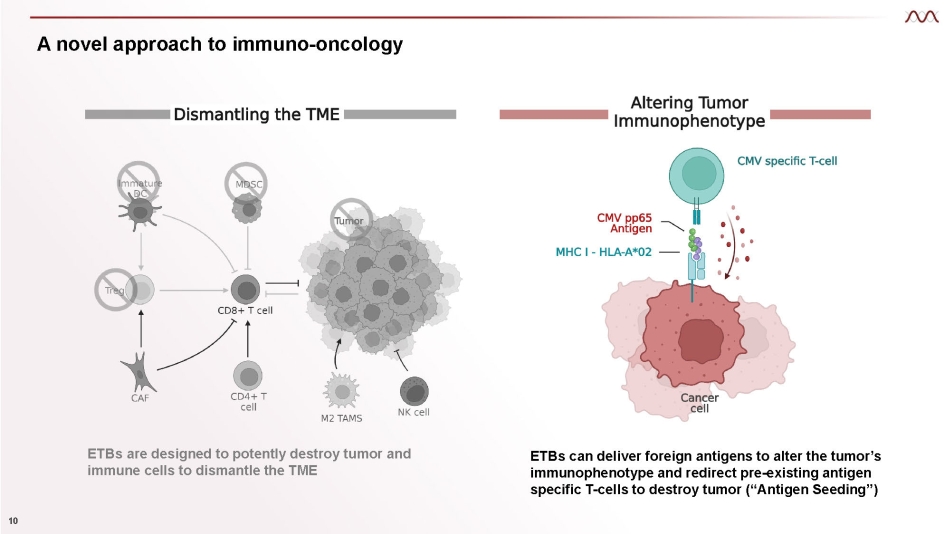
A novel approach to immuno - oncology ETBs are designed to potently destroy tumor and immune cells to dismantle the TME ETBs can deliver foreign antigens to alter the tumor’s immunophenotype and redirect pre - existing antigen specific T - cells to destroy tumor (“Antigen Seeding”) 10

MT - 6402: A novel approach to PD - L1 Part 1 – Altering tumor immunophenotype
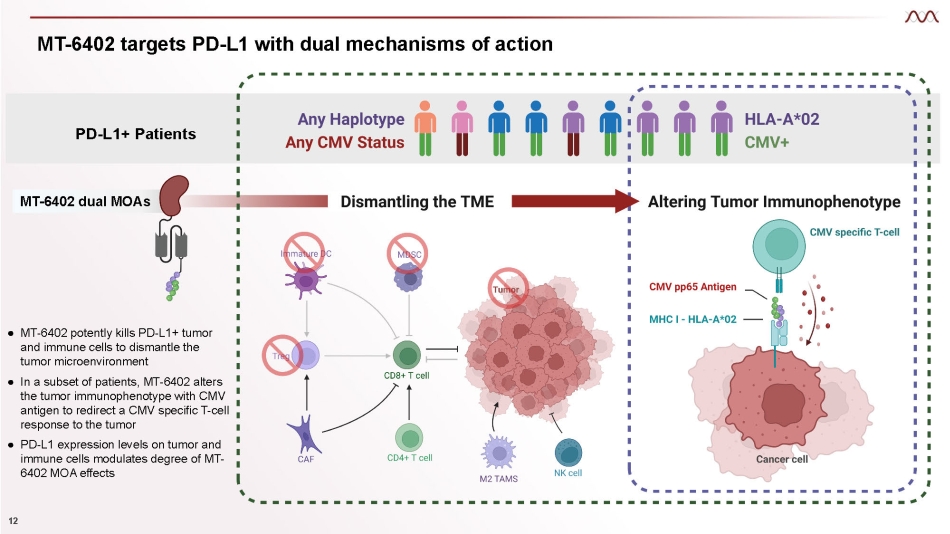
MT - 6402 targets PD - L1 with dual mechanisms of action 12 PD - L1+ Patients ● MT - 6402 potently kills PD - L1+ tumor and immune cells to dismantle the tumor microenvironment ● In a subset of patients, MT - 6402 alters the tumor immunophenotype with CMV antigen to redirect a CMV specific T - cell response to the tumor ● PD - L 1 expression levels on tumor and immune cells modulates degree of MT - 6402 MOA effects MT - 6402 dual MOAs

MT - 6402 clinical overview 13 ● Four dose escalation cohorts completed (16, 24, 32, and 42 mcg/kg) – Patients with any solid tumor type expressing any level of PD - L1 on either the tumor or immune cells – Patients do not have to be CMV+ and/or HLA - A*02 – Patients with a tumor type indicated for checkpoint therapy must have progressed after checkpoint therapy – 63 mcg/kg dose cohort currently enrolling ● Immune related AE’s of grade 2 severity observed (CRS, fever, IRR, rash) – One Grade 3 event of back pain during infusion at 16 mcg/kg (treated with ibuprofen), considered a manifestation of an IRR; one grade 3 IRR at 63 mcg/kg – One Grade 3 event of elevated lipase/amylase at 42 mcg/kg in the setting of intra - abdominal disease progression and porta hepatis compression – One Grade 2 rash at 24 mcg/kg ● Clinical efficacy in an evaluable patient at 16 mcg/kg with near resolution of NSCLC osseous metastases with duration on treatment of approximately 8 months
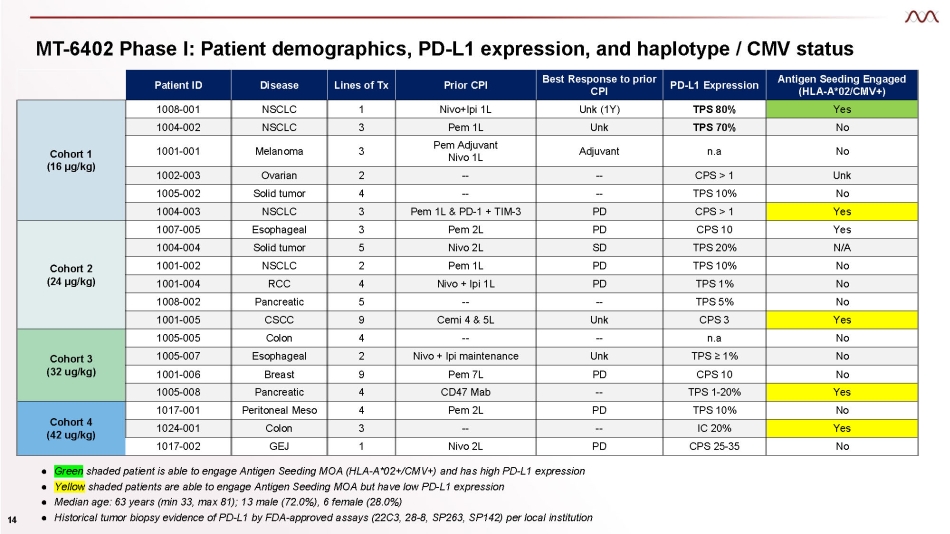
MT - 6402 Phase I: Patient demographics, PD - L1 expression, and haplotype / CMV status Patient ID Disease Lines of Tx Prior CPI Best Response to prior CPI PD - L1 Expression Antigen Seeding Engaged (HLA - A*02/CMV+) Cohort 1 (16 µg/kg) 1008 - 001 NSCLC 1 Nivo+Ipi 1L Unk (1Y) TPS 80% Yes 1004 - 002 NSCLC 3 Pem 1L Unk TPS 70% No 1001 - 001 Melanoma 3 Pem Adjuvant Nivo 1L Adjuvant n.a No 1002 - 003 Ovarian 2 - - - - CPS > 1 Unk 1005 - 002 Solid tumor 4 - - - - TPS 10% No 1004 - 003 NSCLC 3 Pem 1L & PD - 1 + TIM - 3 PD CPS > 1 Yes Cohort 2 (24 µg/kg) 1007 - 005 Esophageal 3 Pem 2L PD CPS 10 Yes 1004 - 004 Solid tumor 5 Nivo 2L SD TPS 20% N/A 1001 - 002 NSCLC 2 Pem 1L PD TPS 10% No 1001 - 004 RCC 4 Nivo + Ipi 1L PD TPS 1% No 1008 - 002 Pancreatic 5 - - - - TPS 5% No 1001 - 005 CSCC 9 Cemi 4 & 5L Unk CPS 3 Yes Cohort 3 (32 ug/kg) 1005 - 005 Colon 4 - - - - n.a No 1005 - 007 Esophageal 2 Nivo + Ipi maintenance Unk TPS ≥ 1% No 1001 - 006 Breast 9 Pem 7L PD CPS 10 No 1005 - 008 Pancreatic 4 CD47 Mab - - TPS 1 - 20% Yes Cohort 4 (42 ug/kg) 1017 - 001 Peritoneal Meso 4 Pem 2L PD TPS 10% No 1024 - 001 Colon 3 - - - - IC 20% Yes 1017 - 002 GEJ 1 Nivo 2L PD CPS 25 - 35 No Green Yellow shaded patient is able to engage Antigen Seeding MOA (HLA - A*02+/CMV+) and has high PD - L1 expression shaded patients are able to engage Antigen Seeding MOA but have low PD - L1 expression Ɣ Ɣ ● Median age: 63 years (min 33, max 81); 13 male (72.0%), 6 female (28.0%) ● Historical tumor biopsy evidence of PD - L1 by FDA - approved assays (22C3, 28 - 8, SP263, SP142) per local institution 14
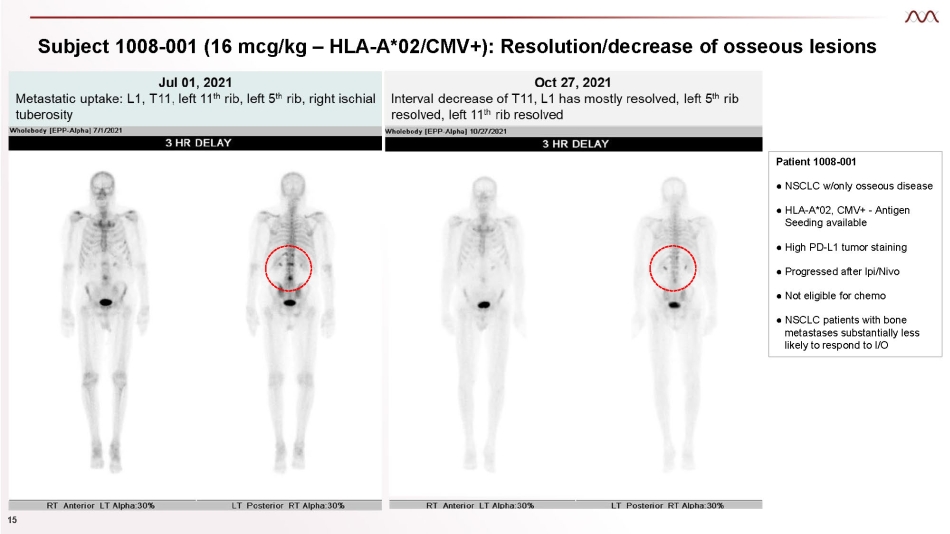
Subject 1008 - 001 (16 mcg/kg – HLA - A*02/CMV+): Resolution/decrease of osseous lesions Patient 1008 - 001 ● NSCLC w/only osseous disease ● HLA - A*02, CMV+ - Antigen Seeding available ● High PD - L1 tumor staining ● Progressed after Ipi/Nivo ● Not eligible for chemo ● NSCLC patients with bone metastases substantially less likely to respond to I/O 15
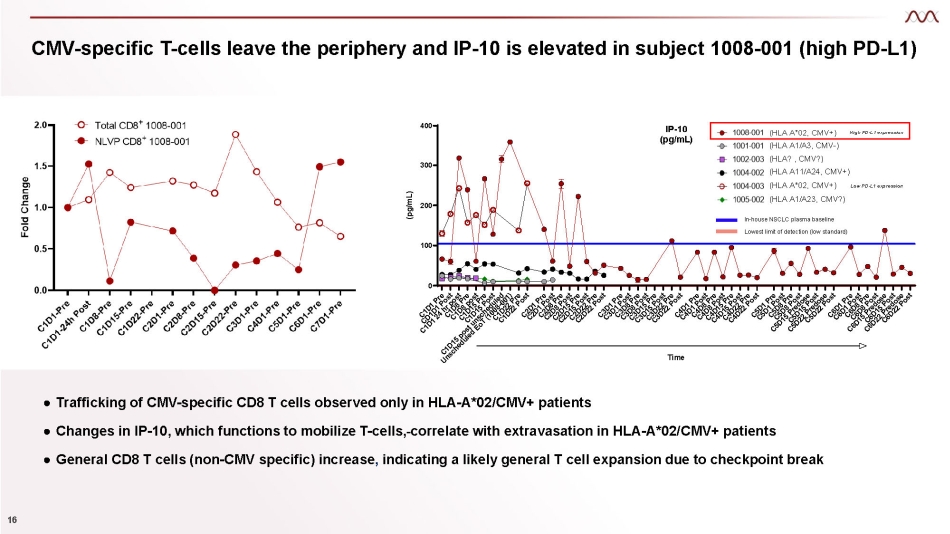
CMV - specific T - cells leave the periphery and IP - 10 is elevated in subject 1008 - 001 (high PD - L1) ● Trafficking of CMV - specific CD8 T cells observed only in HLA - A*02/CMV+ patients ● Changes in IP - 10, which functions to mobilize T - cells, correlate with extravasation in HLA - A*02/CMV+ patients ● General CD8 T cells (non - CMV specific) increase , indicating a likely general T cell expansion due to checkpoint break 0 100 200 300 400 IP - 10 (pg/mL) Time (pg/mL) In - house NSCLC plasma baseline Lowest limit of detection (low standard) (HLA A*02, CMV+) High PD - L1 expression (HLA A1/A3, CMV - ) (HLA? , CMV?) (HLA A11/A24, CMV+) (HLA A*02, CMV+) Low PD - L1 expression (HLA A1/A23, CMV?) 16

MT - 6402: A novel approach to PD - L1 Part 2 – Dismantling the TME
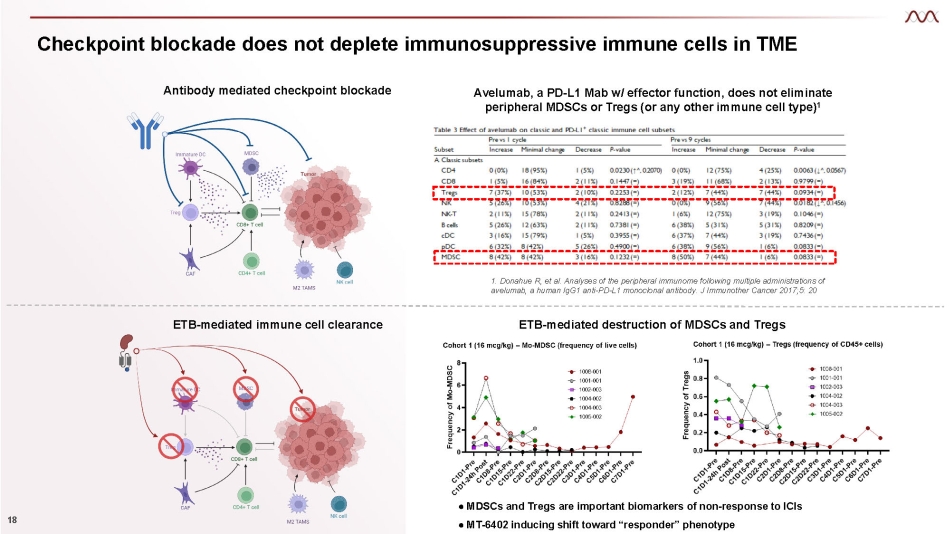
Checkpoint blockade does not deplete immunosuppressive immune cells in TME 18 Antibody mediated checkpoint blockade Avelumab, a PD - L1 Mab w/ effector function, does not eliminate peripheral MDSCs or Tregs (or any other immune cell type) 1 1. Donahue R, et al. Analyses of the peripheral immunome following multiple administrations of avelumab, a human IgG1 anti - PD - L1 monoclonal antibody. J Immunother Cancer 2017;5: 20 ETB - mediated immune cell clearance ● MDSCs and Tregs are important biomarkers of non - response to ICIs ● MT - 6402 inducing shift toward “responder” phenotype ETB - mediated destruction of MDSCs and Tregs
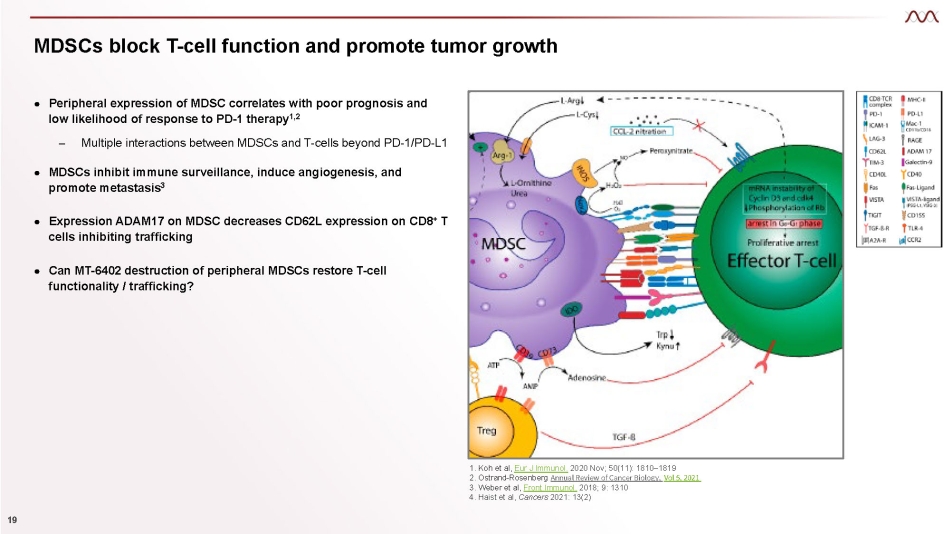
MDSCs block T - cell function and promote tumor growth 1. Koh et al, Eur J Immunol. 2020 Nov; 50(11): 1810 – 1819 2. Ostrand - Rosenberg Annual Review of Cancer Biology, Vol 5, 2021 3. Weber et al, Front Immunol. 2018; 9: 1310 4. Haist et al, Cancers 2021: 13(2) ● Peripheral expression of MDSC correlates with poor prognosis and low likelihood of response to PD - 1 therapy 1,2 – Multiple interactions between MDSCs and T - cells beyond PD - 1/PD - L1 ● MDSCs inhibit immune surveillance, induce angiogenesis, and promote metastasis 3 ● Expression ADAM17 on MDSC decreases CD62L expression on CD8 + T cells inhibiting trafficking ● Can MT - 6402 destruction of peripheral MDSCs restore T - cell functionality / trafficking? 19
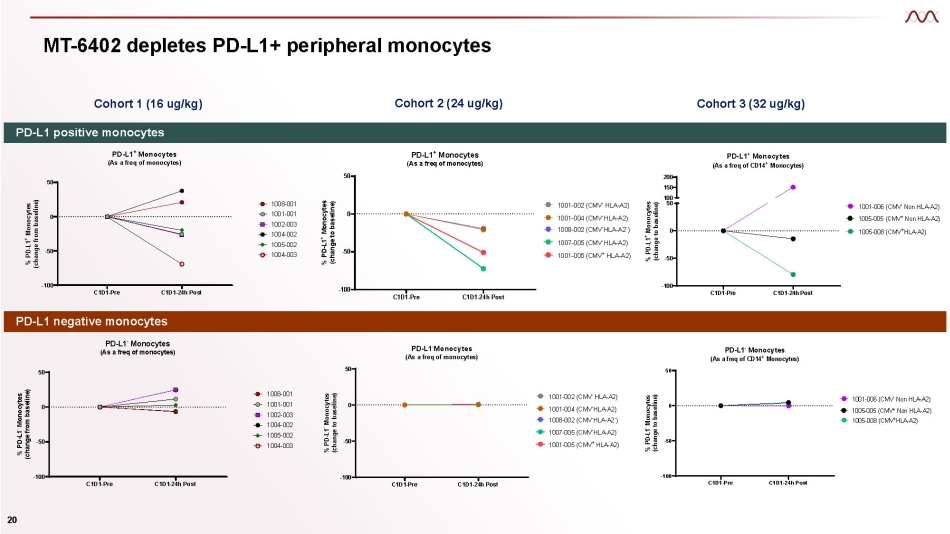
MT - 6402 depletes PD - L1+ peripheral monocytes Cohort 1 (16 ug/kg) Cohort 3 (32 ug/kg) Cohort 2 (24 ug/kg) C1D1 - Pre C1D1 - 24h Post - 100 - 50 0 50 PD - L1 - Monocytes (As a freq of monocytes) % PD - L1 - Monocytes (change from baseline) 1008 - 001 1001 - 001 1002 - 003 1004 - 002 1005 - 002 1004 - 003 C1D1 - Pre C1D1 - 24h Post - 100 - 50 0 50 - PD - L1 Monocytes (As a freq of monocytes) % PD - L1 - Monocytes (change to baseline) 1001 - 002 (CMV - HLA - A2) 1001 - 004 (CMV - HLA - A2) 1008 - 002 (CMV - HLA - A2 - ) 1007 - 005 (CMV - HLA - A2) 1001 - 005 (CMV + HLA - A2) C1D1 - Pre C1D1 - 24h Post - 100 - 50 0 50 PD - L1 - Monocytes (As a freq of CD14 + Monocytes) % PD - L1 - Monocytes (change to baseline) 1001 - 006 (CMV - Non HLA - A2) 1005 - 005 (CMV + Non HLA - A2) 1005 - 008 (CMV + HLA - A2) PD - L1 positive monocytes PD - L1 negative monocytes C1D1 - Pre C1D1 - 24h Post - 100 - 50 0 50 PD - L1 + Monocytes (As a freq of monocytes) % PD - L1 + Monocytes (change from baseline) 1008 - 001 1001 - 001 1002 - 003 1004 - 002 1005 - 002 1004 - 003 C1D1 - Pre C1D1 - 24h Post - 100 - 50 0 50 PD - L1 + Monocytes (As a freq of monocytes) % PD - L1 + Monocytes (change to baseline) - - 1001 - 002 (CMV - HLA - A2) 1001 - 004 (CMV - HLA - A2) 1008 - 002 (CMV HLA - A2 ) 1007 - 005 (CMV - HLA - A2) 1001 - 005 (CMV + HLA - A2) C1D1 - Pre C1D1 - 24h Post - 100 - 50 0 200 150 100 50 PD - L1 + Monocytes (As a freq of CD14 + Monocytes) % PD - L1 + Monocytes (change to baseline) 1001 - 006 (CMV - Non HLA - A2) 1005 - 005 (CMV + Non HLA - A2) 1005 - 008 (CMV + HLA - A2) 20
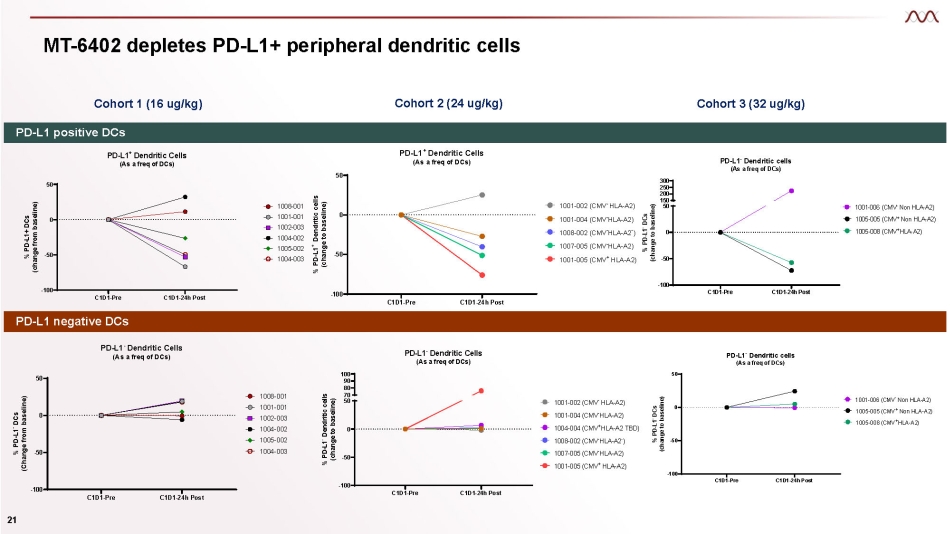
MT - 6402 depletes PD - L1+ peripheral dendritic cells Cohort 1 (16 ug/kg) Cohort 3 (32 ug/kg) Cohort 2 (24 ug/kg) PD - L1 positive DCs PD - L1 negative DCs C1D1 - Pre C1D1 - 24h Post - 100 - 50 0 50 PD - L1 + Dendritic Cells (As a freq of DCs) % PD - L1+ DCs (change from baseline) 1008 - 001 1001 - 001 1002 - 003 1004 - 002 1005 - 002 1004 - 003 C1D1 - Pre C1D1 - 24h Post - 100 - 50 0 50 PD - L1 + Dendritic Cells (As a freq of DCs) % PD - L1 + Dendritic cells (change to baseline) - 1001 - 002 (CMV HLA - A2) 1001 - 004 (CMV - HLA - A2) 1008 - 002 (CMV - HLA - A2 - ) 1007 - 005 (CMV - HLA - A2) 1001 - 005 (CMV + HLA - A2) C1D1 - Pre C1D1 - 24h Post - 100 - 50 0 300 250 200 150 50 PD - L1 - Dendritic cells (As a freq of DCs) % PD - L1 - DCs (change to baseline) - 1001 - 006 (CMV Non HLA - A2) 1005 - 005 (CMV + Non HLA - A2) 1005 - 008 (CMV + HLA - A2) C1D1 - Pre C1D1 - 24h Post - 100 - 50 0 50 PD - L1 - Dendritic Cells (As a freq of DCs) % PD - L1 - DCs (Change from baseline) 1008 - 001 1001 - 001 1002 - 003 1004 - 002 1005 - 002 1004 - 003 C1D1 - Pre C1D1 - 24h Post - 100 - 50 0 100 90 80 70 50 - PD - L1 Dendritic Cells (As a freq of DCs) % PD - L1 - Dendritic cells (change to baseline) - 1001 - 002 (CMV HLA - A2) 1001 - 004 (CMV - HLA - A2) 1004 - 004 (CMV + HLA - A2 TBD) 1008 - 002 (CMV - HLA - A2 - ) 1007 - 005 (CMV - HLA - A2) 1001 - 005 (CMV + HLA - A2) C1D1 - Pre C1D1 - 24h Post - 100 - 50 0 50 PD - L1 - Dendritic cells (As a freq of DCs) % PD - L1 - DCs (change to baseline) 1001 - 006 (CMV - Non HLA - A2) 1005 - 005 (CMV + Non HLA - A2) 1005 - 008 (CMV + HLA - A2) 21
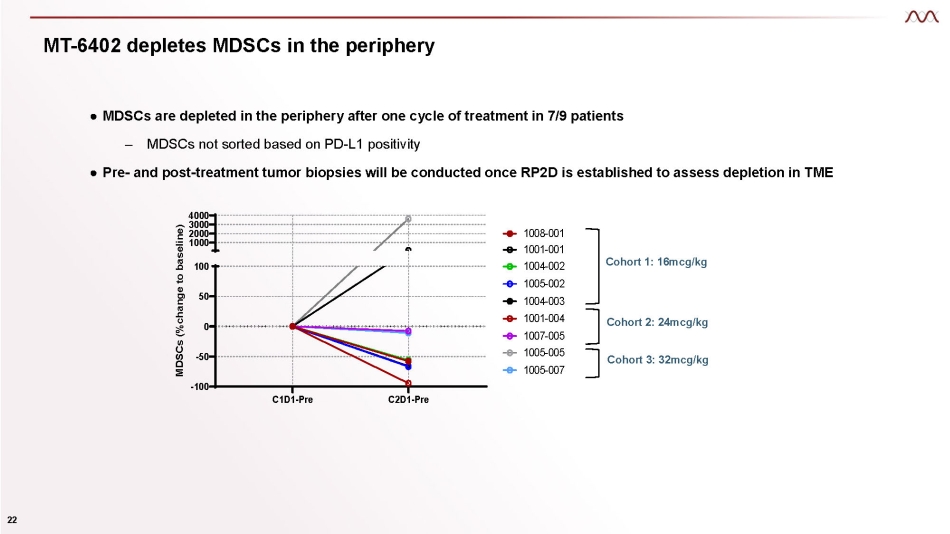
MT - 6402 depletes MDSCs in the periphery ● MDSCs are depleted in the periphery after one cycle of treatment in 7/9 patients – MDSCs not sorted based on PD - L1 positivity ● Pre - and post - treatment tumor biopsies will be conducted once RP2D is established to assess depletion in TME C1D1 - Pre C2D1 - Pre - 100 - 50 0 50 100 4000 3000 2000 1000 MDSCs (%change to baseline) 22 1008 - 001 1001 - 001 1004 - 002 1005 - 002 1004 - 003 1001 - 004 1007 - 005 1005 - 005 1005 - 007 Cohort 1: 16mcg/kg Cohort 3: 32mcg/kg Cohort 2: 24mcg/kg
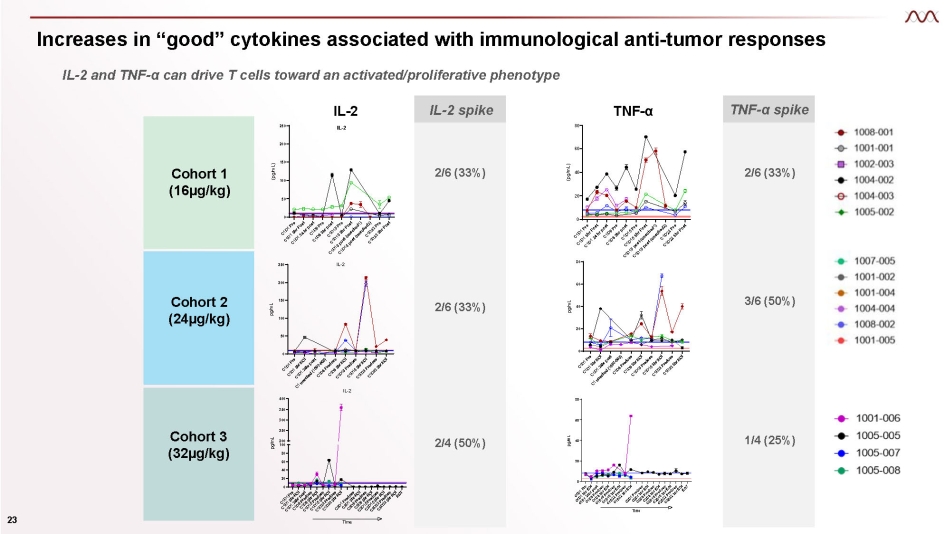
Increases in “good” cytokines associated with immunological anti - tumor responses IL - 2 and TNF - α can drive T cells toward an activated/proliferative phenotype 0 20 40 60 80 pg/mL TNF - α 0 50 100 150 200 250 IL - 2 pg/mL 0 20 40 60 80 TNF - α (pg/mL) Cohort 1 (16µg/kg) Cohort 2 (24µg/kg) 0 20 40 60 80 Time pg/mL TNFa Cohort 3 (32µg/kg) IL - 2 spike 2/6 (33%) 2/6 (33%) 2/4 (50%) TNF - α spike 2/6 (33%) 3/6 (50%) 1/4 (25%) TNF - α 0 50 100 150 200 250 IL - 2 IL - 2 (pg/mL) 20 0 60 40 200 100 80 250 300 350 400 IL - 2 TIme pg/mL 23
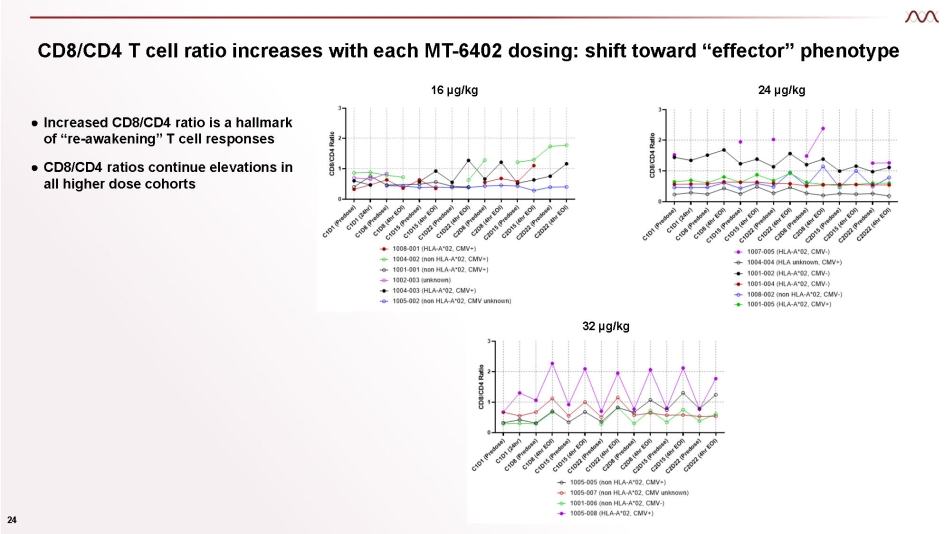
CD8/CD4 T cell ratio increases with each MT - 6402 dosing: shift toward “effector” phenotype 16 µg/kg 24 µg/kg 32 µg/kg 24 ● Increased CD8/CD4 ratio is a hallmark of “re - awakening” T cell responses ● CD8/CD4 ratios continue elevations in all higher dose cohorts
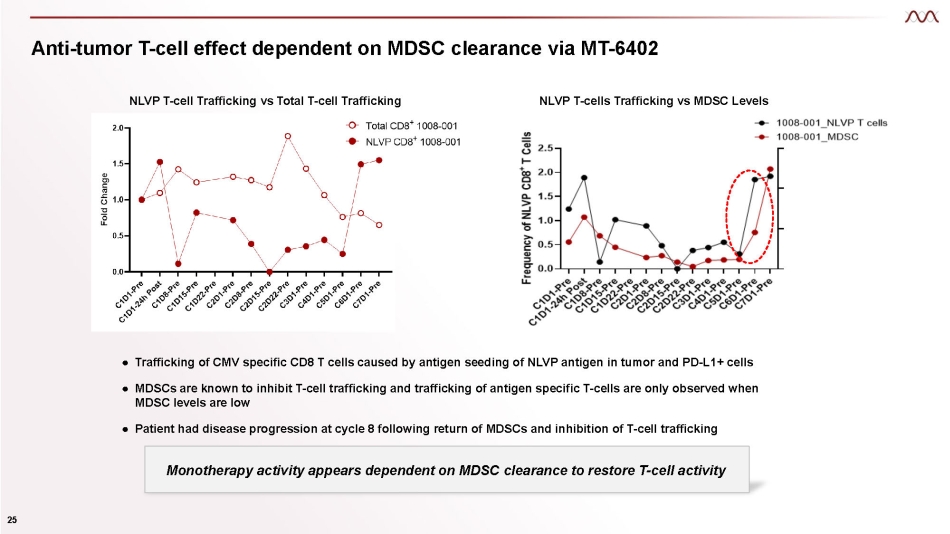
Anti - tumor T - cell effect dependent on MDSC clearance via MT - 6402 ● Trafficking of CMV specific CD8 T cells caused by antigen seeding of NLVP antigen in tumor and PD - L1+ cells ● MDSCs are known to inhibit T - cell trafficking and trafficking of antigen specific T - cells are only observed when MDSC levels are low ● Patient had disease progression at cycle 8 following return of MDSCs and inhibition of T - cell trafficking NLVP T - cells Trafficking vs MDSC Levels 25 NLVP T - cell Trafficking vs Total T - cell Trafficking Monotherapy activity appears dependent on MDSC clearance to restore T - cell activity
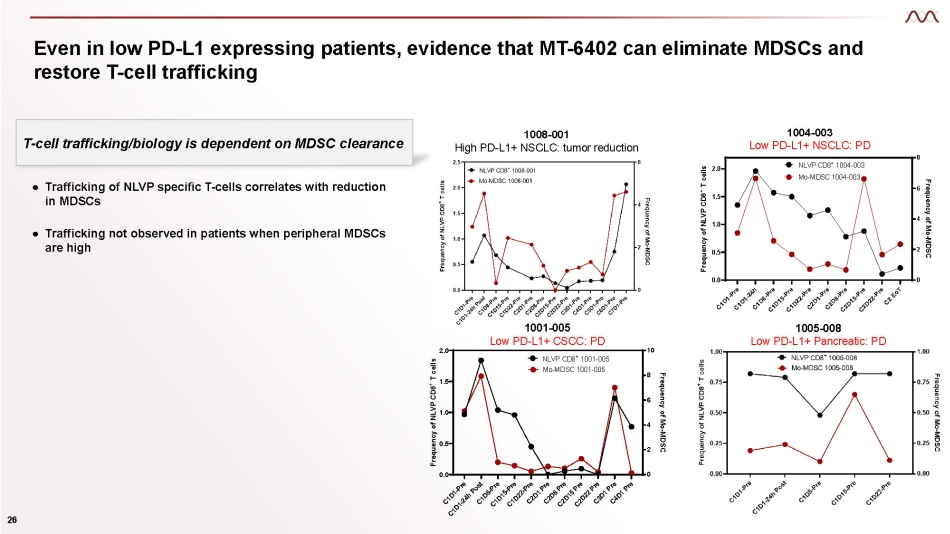
Even in low PD - L1 expressing patients, evidence that MT - 6402 can eliminate MDSCs and restore T - cell trafficking 1005 - 008 Low PD - L1+ Pancreatic: PD 0.0 0.5 1.0 1.5 2.0 0 2 4 6 8 Frequency of NLVP CD8 + T cells Frequency of Mo - MDSC NLVP CD8 + 1004 - 003 Mo - MDSC 1004 - 003 0.0 0.5 1.0 1.5 2.0 0 2 4 6 8 10 Frequency of NLVP CD8 + T cells Frequency of Mo - MDSC NLVP CD8 + 1001 - 005 Mo - MDSC 1001 - 005 ● Trafficking of NLVP specific T - cells correlates with reduction in MDSCs 26 ● Trafficking not observed in patients when peripheral MDSCs are high 1001 - 005 Low PD - L1+ CSCC: PD T - cell trafficking/biology is dependent on MDSC clearance 1008 - 001 High PD - L1+ NSCLC: tumor reduction 1004 - 003 Low PD - L1+ NSCLC: PD
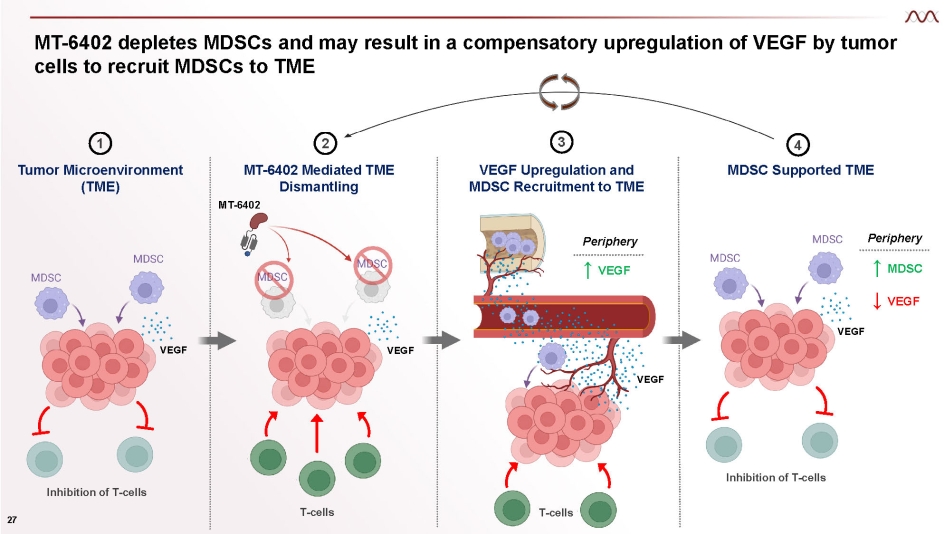
MT - 6402 depletes MDSCs and may result in a compensatory upregulation of VEGF by tumor cells to recruit MDSCs to TME Inhibition of T - cells T - cells VEGF Upregulation and MDSC Recruitment to TME VEGF VEGF ↑ VEGF Inhibition of T - cells Periphery T - cells ↑ MDSC ↓ VEGF Periphery 1 Tumor Microenvironment (TME) 3 4 MDSC Supported TME VEGF 27 VEGF 2 MT - 6402 Mediated TME Dismantling MT - 6402
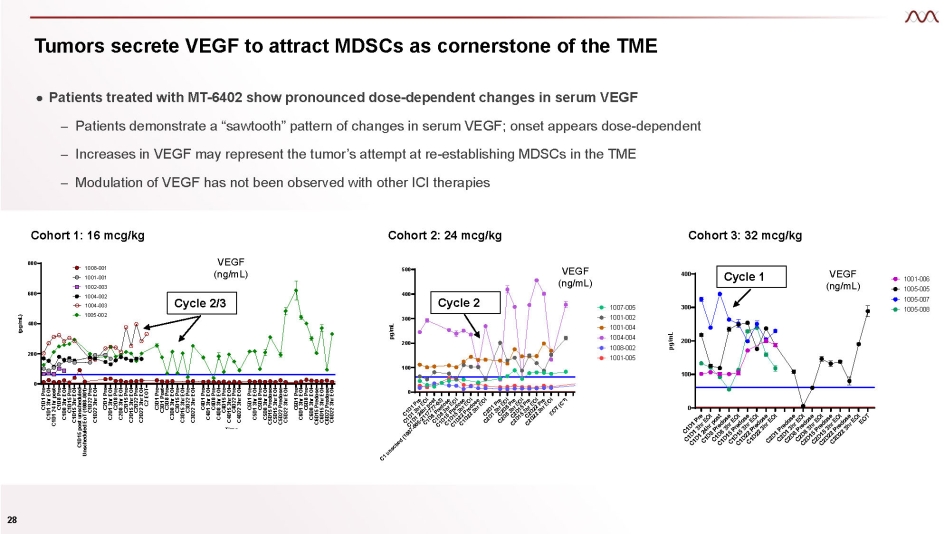
Tumors secrete VEGF to attract MDSCs as cornerstone of the TME ● Patients treated with MT - 6402 show pronounced dose - dependent changes in serum VEGF – Patients demonstrate a “sawtooth” pattern of changes in serum VEGF; onset appears dose - dependent – Increases in VEGF may represent the tumor’s attempt at re - establishing MDSCs in the TME – Modulation of VEGF has not been observed with other ICI therapies Cohort 1: 16 mcg/kg C1D1 Pre C1D1 3hr EOI C1D1 24 hr post C1D8 Pre C1D8 3hr EOI C1D15 Pre C1D15 3hr EOI C1D15 post unscheduled Unscheduled EoT (1008 - 001) C1D22 Pre C1D22 3hr EOI C2D1 Pre C2D1 3hr EOI C2D8 Pre C2D8 3hr EOI C2D15 Pre C2D15 3hr EOI C2D22 Pre C2D22 3hr EOI C2 EOT C3D1 Pre C3D1 Post C3D8 Pre C3D8 3hr EOI C3D15 Pre C3D15 3hr EOI C3D22 Pre C3D22 3hr EOI C4D1 Pre C4D1 3hr EOI C4D8 Pre C4D8 3hr EOI C4D15 Pre C4D15 3hr EOI C4D22 Pre C4D22 3hr EOI C5D1 Pre C5D1 3hr EOI C5D8 Pre C5D8 3hr EOI C5D15 Predose C5D15 3hr EOI C5D22 Predose C5D22 3hr EOI C6D1 Pre C6D1 3hr EOI C6D8 Pre C6D8 3hr EOI C6D15 Predose C6D15 3hr EOI C6D22 Predose C6D22 3hr EOI 0 200 400 600 800 VEGF (pg/mL) Time (pg/mL) 1008 - 001 1001 - 001 1002 - 003 1004 - 002 1004 - 003 1005 - 002 Cohort 3: 32 mcg/kg VEGF (ng/mL) 0 100 200 300 400 Time pg/mL 1001 - 006 1005 - 005 1005 - 007 1005 - 008 Cohort 2: 24 mcg/kg VEGF (ng/mL) 0 100 200 300 400 500 Time pg/mL 1007 - 005 1001 - 002 1001 - 004 1004 - 004 1008 - 002 1001 - 005 Cycle 2/3 Cycle 2 Cycle 1 VEGF (ng/mL) 28

MT - 8421 ETB with novel MOA targeting CTLA - 4
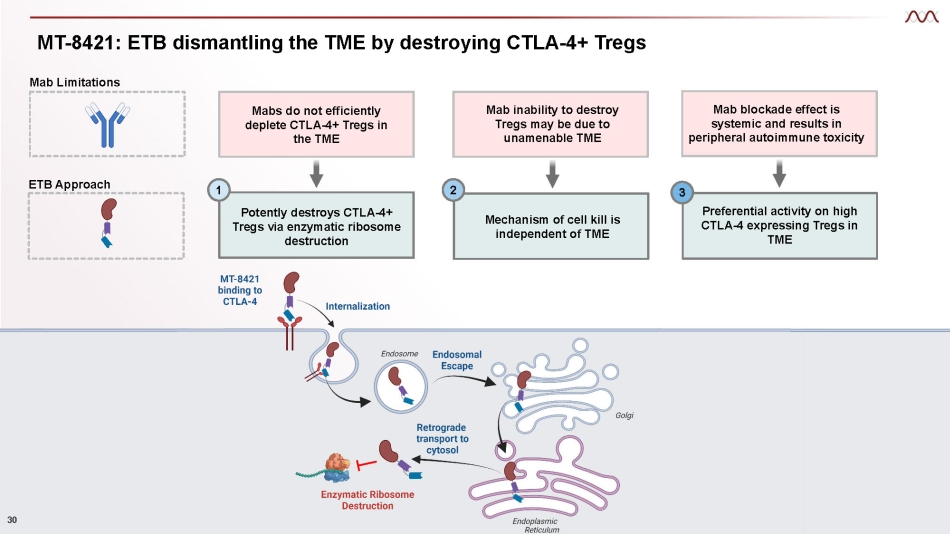
MT - 8421: ETB dismantling the TME by destroying CTLA - 4+ Tregs Potently destroys CTLA - 4+ Tregs via enzymatic ribosome destruction Mechanism of cell kill is independent of TME Preferential activity on high CTLA - 4 expressing Tregs in TME 1 2 3 Mab Limitations Broad CTLA - 4 blockade effect causes autoimmune toxicity Mab inability to destroy Tregs may be due to unamenable TME Mab blockade effect is systemic and results in peripheral autoimmune toxicity Mabs do not efficiently deplete CTLA - 4+ Tregs in the TME ETB Approach 30
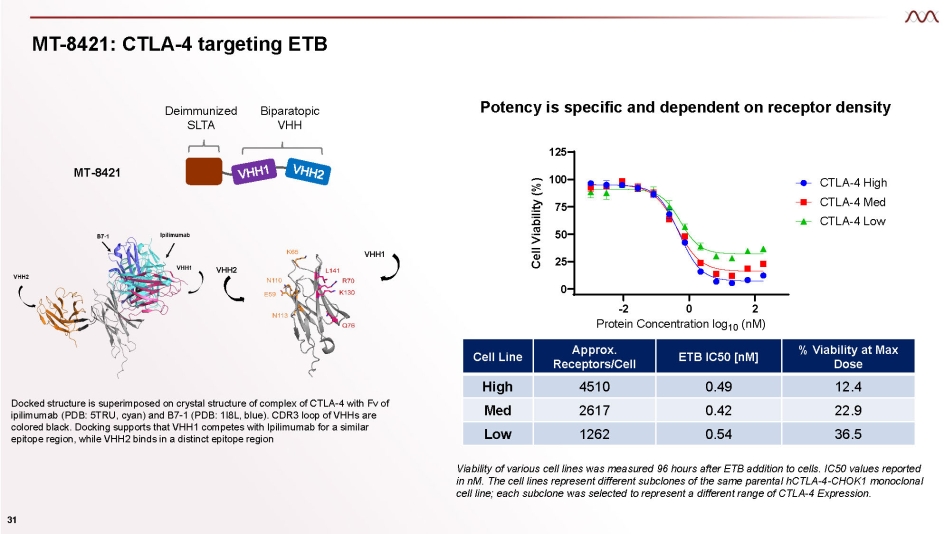
MT - 8421: CTLA - 4 targeting ETB MT - 8421 Deimmunized SLTA Biparatopic VHH Docked structure is superimposed on crystal structure of complex of CTLA - 4 with Fv of ipilimumab (PDB: 5TRU, cyan) and B7 - 1 (PDB: 1I8L, blue). CDR3 loop of VHHs are colored black. Docking supports that VHH1 competes with Ipilimumab for a similar epitope region, while VHH2 binds in a distinct epitope region VHH1 VHH2 Cell Line Approx. Receptors/Cell ETB IC50 [nM] % Viability at Max Dose High 4510 0.49 12.4 Med 2617 0.42 22.9 Low 1262 0.54 36.5 Viability of various cell lines was measured 96 hours after ETB addition to cells . IC 50 values reported in nM . The cell lines represent different subclones of the same parental hCTLA - 4 - CHOK 1 monoclonal cell line ; each subclone was selected to represent a different range of CTLA - 4 Expression . 25 50 75 100 125 CTG2.0 Viability 4 - day Assay 0 - 2 0 2 Protein Concentration log 10 (nM) Cell Viability (%) CTLA - 4 High CTLA - 4 Med CTLA - 4 Low 31 Potency is specific and dependent on receptor density
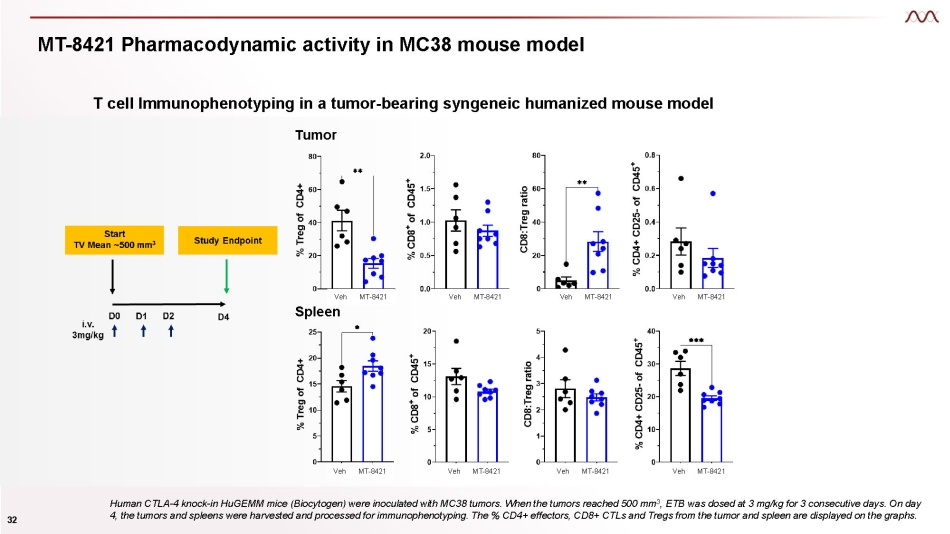
MT - 8421 Pharmacodynamic activity in MC38 mouse model T cell Immunophenotyping in a tumor - bearing syngeneic humanized mouse model Tumor Human CTLA - 4 knock - in HuGEMM mice (Biocytogen) were inoculated with MC38 tumors. When the tumors reached 500 mm 3 , ETB was dosed at 3 mg/kg for 3 consecutive days. On day 4, the tumors and spleens were harvested and processed for immunophenotyping. The % CD4+ effectors, CD8+ CTLs and Tregs from the tumor and spleen are displayed on the graphs. Veh Spleen MT - 8421 Veh MT - 8421 Veh MT - 8421 Veh MT - 8421 Veh MT - 8421 Veh MT - 8421 Veh MT - 8421 Veh MT - 8421 32

MT - 5111 ETB with novel MOA targeting HER2
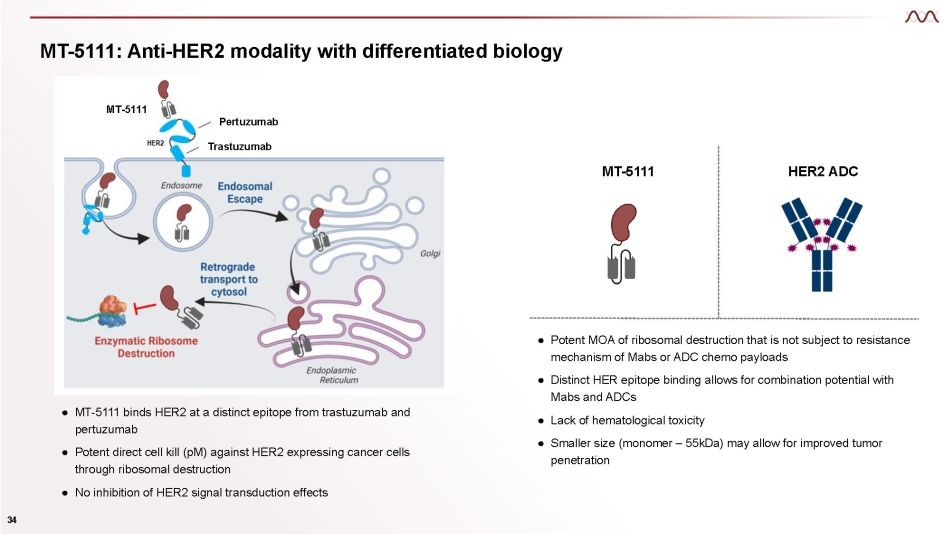
MT - 5111: Anti - HER2 modality with differentiated biology ● MT - 5111 binds HER2 at a distinct epitope from trastuzumab and pertuzumab ● Potent direct cell kill (pM) against HER2 expressing cancer cells through ribosomal destruction ● No inhibition of HER2 signal transduction effects ● Potent MOA of ribosomal destruction that is not subject to resistance mechanism of Mabs or ADC chemo payloads ● Distinct HER epitope binding allows for combination potential with Mabs and ADCs ● Lack of hematological toxicity ● Smaller size (monomer – 55kDa) may allow for improved tumor penetration MT - 5111 HER2 ADC Pertuzumab Trastuzumab MT - 5111 34
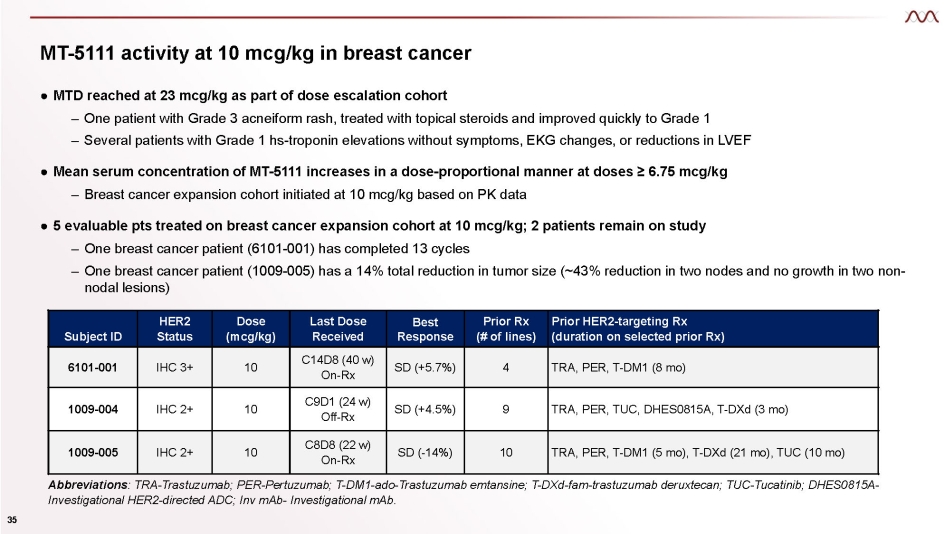
MT - 5111 activity at 10 mcg/kg in breast cancer 35 ● MTD reached at 23 mcg/kg as part of dose escalation cohort – One patient with Grade 3 acneiform rash, treated with topical steroids and improved quickly to Grade 1 – Several patients with Grade 1 hs - troponin elevations without symptoms, EKG changes, or reductions in LVEF ● Mean serum concentration of MT - 5111 increases in a dose - proportional manner at doses ≥ 6.75 mcg/kg – Breast cancer expansion cohort initiated at 10 mcg/kg based on PK data ● 5 evaluable pts treated on breast cancer expansion cohort at 10 mcg/kg; 2 patients remain on study – One breast cancer patient (6101 - 001) has completed 13 cycles – One breast cancer patient (1009 - 005) has a 14% total reduction in tumor size (~43% reduction in two nodes and no growth in two non - nodal lesions) Subject ID HER2 Status Dose (mcg/kg) Last Dose Received Best Response Prior Rx (# of lines) Prior HER2 - targeting Rx (duration on selected prior Rx) 6101 - 001 IHC 3+ 10 C14D8 (40 w) On - Rx SD (+5.7%) 4 TRA, PER, T - DM1 (8 mo) 1009 - 004 IHC 2+ 10 C9D1 (24 w) Off - Rx SD (+4.5%) 9 TRA, PER, TUC, DHES0815A, T - DXd (3 mo) 1009 - 005 IHC 2+ 10 C8D8 (22 w) On - Rx SD ( - 14%) 10 TRA, PER, T - DM1 (5 mo), T - DXd (21 mo), TUC (10 mo) Abbreviations : TRA - Trastuzumab; PER - Pertuzumab; T - DM1 - ado - Trastuzumab emtansine; T - DXd - fam - trastuzumab deruxtecan; TUC - Tucatinib; DHES0815A - Investigational HER2 - directed ADC; Inv mAb - Investigational mAb.
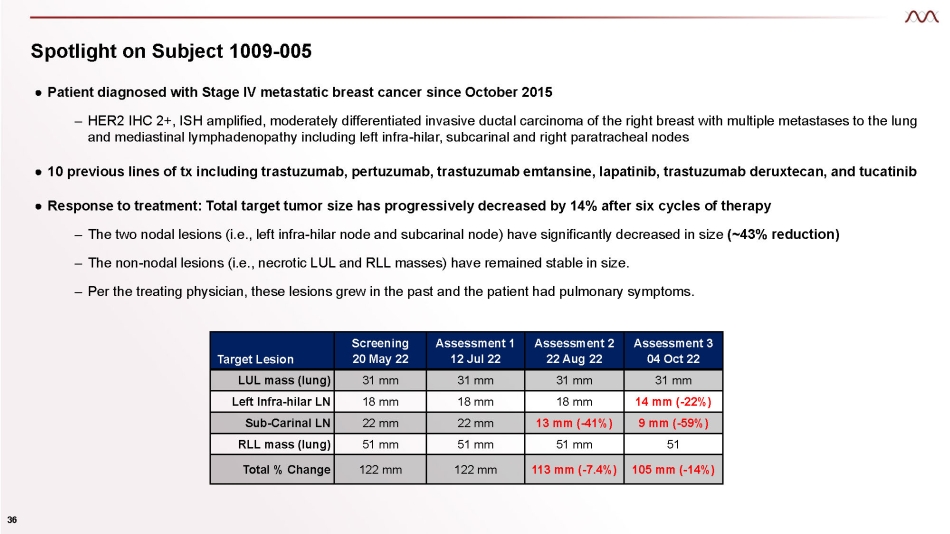
Spotlight on Subject 1009 - 005 36 ● Patient diagnosed with Stage IV metastatic breast cancer since October 2015 – HER2 IHC 2+, ISH amplified, moderately differentiated invasive ductal carcinoma of the right breast with multiple metastases to the lung and mediastinal lymphadenopathy including left infra - hilar, subcarinal and right paratracheal nodes ● 10 previous lines of tx including trastuzumab, pertuzumab, trastuzumab emtansine, lapatinib, trastuzumab deruxtecan, and tucatinib ● Response to treatment: Total target tumor size has progressively decreased by 14% after six cycles of therapy – The two nodal lesions (i.e., left infra - hilar node and subcarinal node) have significantly decreased in size (~43% reduction) – The non - nodal lesions (i.e., necrotic LUL and RLL masses) have remained stable in size. – Per the treating physician, these lesions grew in the past and the patient had pulmonary symptoms. Target Lesion Screening 20 May 22 Assessment 1 12 Jul 22 Assessment 2 22 Aug 22 Assessment 3 04 Oct 22 LUL mass (lung) 31 mm 31 mm 31 mm 31 mm Left Infra - hilar LN 18 mm 18 mm 18 mm 14 mm ( - 22%) Sub - Carinal LN 22 mm 22 mm 13 mm ( - 41%) 9 mm ( - 59%) RLL mass (lung) 51 mm 51 mm 51 mm 51 Total % Change 122 mm 122 mm 113 mm ( - 7.4%) 105 mm ( - 14%)
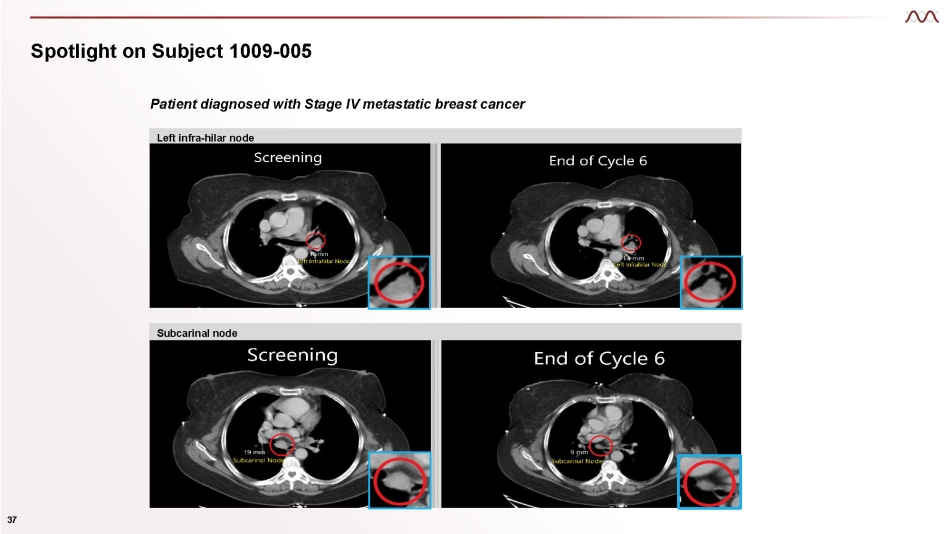
Spotlight on Subject 1009 - 005 Patient diagnosed with Stage IV metastatic breast cancer Left infra - hilar node Subcarinal node 37

MT - 0169 ETB with novel MOA targeting CD38
Initial MT - 0169 starting dose of 50 mcg/kg exceeded therapeutic window 39 ● Five heavily pretreated R - R MM patients were treated at the initial Phase I starting dose of 50 mcg/kg – Highest starting dose of any ETB clinical program ● Two patients had asymptomatic and reversible cardiac DLT’s – Grade 2 reversible myocarditis and Grade 3 reversible non - ischemic cardiomyopathy – All patients demonstrated very rapid clearance of CD38+ NK cells within hours of 1 st dose of MT - 0169 – CD38 expressed at low levels on myocardial endothelial cells; high starting dose may have inadvertently targeted myocardial endothelial cells ● Only one patient in 50 mcg/kg was evaluable for response and had PD – Two of the patients were non - evaluable due to DLTs, and two patients progressed during cycle 1 ● Five patients tested at 50 mcg/kg not sufficient to assess utility of forced internalization and novel MOA targeting CD38
MT - 0169 Phase I restarted at 5 mcg/kg with one of four patients in a clinical response 40 ● Four heavily pre - treated R/R MM patients have been treated at 5 mcg/kg – Additional tests added to the protocol to extend cardiac safety monitoring ● No toxicity observed at 5 mcg/kg cohort; 10 mcg/kg cohort is enrolling ● CD38+ NK cell depletion noted but less profound and rapid than what was observed at 50 mcg/kg ● One patient remains on treatment with a partial response at end of cycle 2; PET scan planned to determine if patient is in a CR – Patient progressed after six lines of therapy including multiple proteasome inhibitors, multiple IMiDs, Dara, and a BCMA/CD3 bispecific – Patient’s lab parameters have improved significantly with serum free light chain lambda and IgA spike showing marked decrease – Serum immunofixation has converted from positive to negative – CRP has improved considerably and likely explains the patient’s improvement from a hemoglobin of 10 g/dL to 14 g/dL ● Patient clinical response suggests therapeutic index is between 5 mcg/kg and 50 mcg/kg

ETB Pipeline and Platform Diversified ETB pipeline with novel MOAs
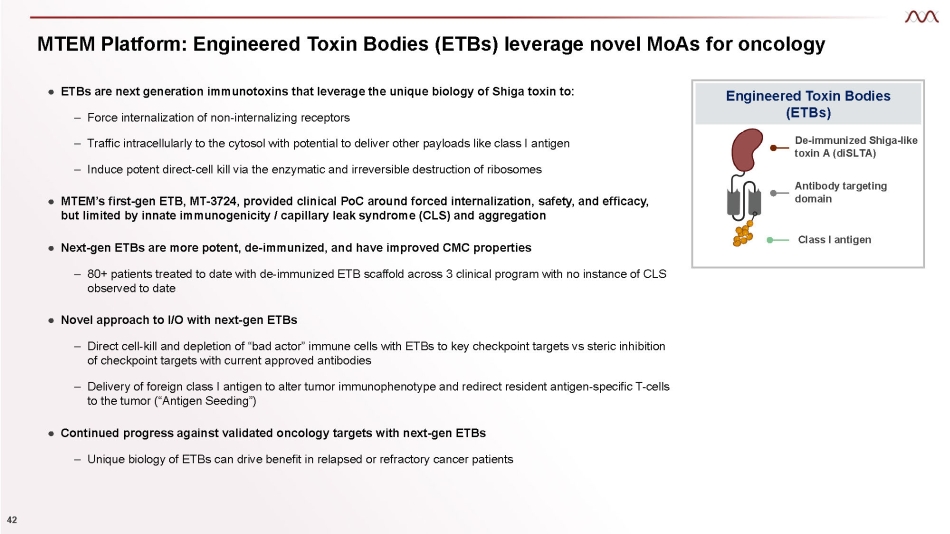
MTEM Platform: Engineered Toxin Bodies (ETBs) leverage novel MoAs for oncology Engineered Toxin Bodies (ETBs) De - immunized Shiga - like toxin A (diSLTA) Antibody targeting domain Class I antigen ● ETBs are next generation immunotoxins that leverage the unique biology of Shiga toxin to: 42 – Force internalization of non - internalizing receptors – Traffic intracellularly to the cytosol with potential to deliver other payloads like class I antigen – Induce potent direct - cell kill via the enzymatic and irreversible destruction of ribosomes ● MTEM’s first - gen ETB, MT - 3724, provided clinical PoC around forced internalization, safety, and efficacy, but limited by innate immunogenicity / capillary leak syndrome (CLS) and aggregation ● Next - gen ETBs are more potent, de - immunized, and have improved CMC properties – 80+ patients treated to date with de - immunized ETB scaffold across 3 clinical program with no instance of CLS observed to date ● Novel approach to I/O with next - gen ETBs – Direct cell - kill and depletion of “bad actor” immune cells with ETBs to key checkpoint targets vs steric inhibition of checkpoint targets with current approved antibodies – Delivery of foreign class I antigen to alter tumor immunophenotype and redirect resident antigen - specific T - cells to the tumor (“Antigen Seeding”) ● Continued progress against validated oncology targets with next - gen ETBs – Unique biology of ETBs can drive benefit in relapsed or refractory cancer patients
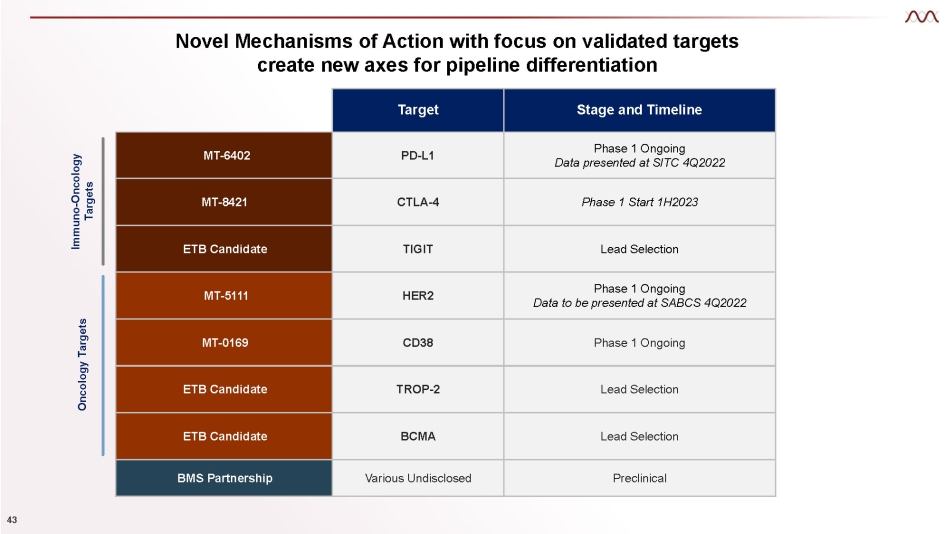
Novel Mechanisms of Action with focus on validated targets create new axes for pipeline differentiation Target Stage and Timeline MT - 6402 PD - L1 Phase 1 Ongoing Data presented at SITC 4Q2022 MT - 8421 CTLA - 4 Phase 1 Start 1H2023 ETB Candidate TIGIT Lead Selection MT - 5111 HER2 Phase 1 Ongoing Data to be presented at SABCS 4Q2022 MT - 0169 CD38 Phase 1 Ongoing ETB Candidate TROP - 2 Lead Selection ETB Candidate BCMA Lead Selection BMS Partnership Various Undisclosed Preclinical Immuno - Oncology Targets Oncology Targets 43